BlinkLab is a world-first, AI-driven digital healthcare venture, that uses a smartphone and facial recognition to detect autism and ADHD.
It’s listing on the ASX next Thursday (April 4) under the code BB1.
BlinkLab’s development is being led by chair Brian Leedman – a founder of ResApp Health – the cough analysis app sold to Pfizer two years ago in a $179 million deal.
Now Brian’s confident that BlinkLab – first developed at Princeton University – could mirror that success, while also fast-tracking detection of Autism and ADHD and improving patient outcomes.
“We’re the only company in the world using computer vision and machine learning to assess brain responses to audio and visual stimulation – delivered via a smartphone – to detect autism and ADHD,” Mr Leedman said.
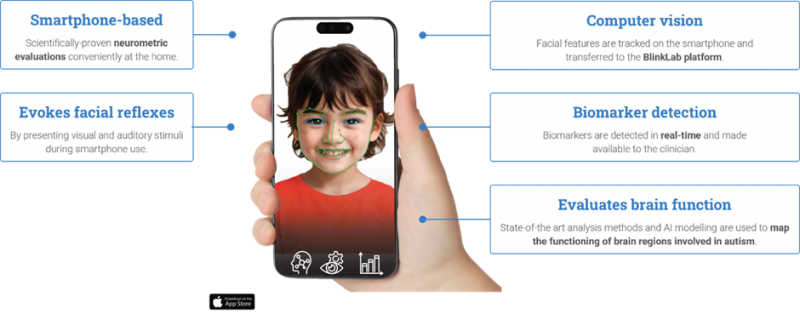
“It’s easy for a doctor or specialist to use as a diagnostic tool. We can diagnose children from 18 months old, which is years younger than through traditional methods that typically result in children being diagnosed from the age of five,” he said.
“By the time a child reaches five years old, neuronal development in the brain has significantly advanced as have the behavioural patterns that led to the concerns of the parents. If the child is autistic, then they were autistic from birth.
“As in all medicines, the earlier you can diagnose anyone with anything, the better the outcomes for the patient.”
There are lengthy diagnosis delays both here in Australia and abroad, so the demand for such a tool is extensive.
The global Autism Spectrum Disorder market is expected to reach $700 billion by 2028
BlinkLab uses AI and machine learning algorithms to predict autism and ADHD and recent clinical trials demonstrated sensitivity of 85% and specificity of 84%, suggesting much higher accuracy comparing to existing FDA approved products.
“Based on clinical trial results to date, we have a very accurate test for autism and we now have to replicate that in a US FDA (Food and Drug Administration) medical device registration study,” Brian Leedman said.
“The NDIS in Australia has come under scrutiny for the tremendous cost to the Australian taxpayer over the fact that ASD (Autism Spectrum Disorder) diagnosis and treatment in children is the single largest expenditure – so the timing for this technology development and ASX listing is spot on.”
Industry data suggests more than a third of those claiming through NDIS report the primary condition of autism. The NDIS paid out $6.73 billion dollars in payments to support those Australians last year alone. What’s also concerning is that the figure was 28 per cent higher than for the previous year, 2022. Autism rates in Australia are among the highest in the world and effect 1 in 25 children.

BlinkLab’s FDA trial will recruit up to 500 people, and to fund it, BB1 is raising $7 million through the IPO process with 20 cent shares. It plans to complete the study by mid-next year with the aim of gaining FDA approval by 2026. The neurometric testing technology is also being developed and studied for its efficacy in screening for schizophrenia and forms of dementia.
Backed by Princeton University
BlinkLab has an exclusive global commercial licence agreement with Princeton University, where Founders Dr Henk-Jan Boele, Peter Boele and Bas Koekkoek first created early iterations of the idea. It’s come a long way since then with $4.4 million spent on the Software as a Medical Device development. There’ve been 6000 individual diagnostic tests already carried out throughout the world, including eight studies as far afield as Morocco and Ecuador.
The company has forged numerous partnerships with credible institutions including Princeton University, Penn Medicine, Erasmus MC, Baylor College of Medicine, and many others.
No safety risk, BlinkLab just has to prove efficacy
Mr Leedman said another advantage in developing medtech such as BlinkLab was that there could be no safety risks or delays.
“The worst that can happen is the phone is dropped on your foot – literally,” he said.
“That means we don’t face the long and slow process of testing for safety, we just have to prove efficacy.”
Aside from his success with ResApp Health, it’s worth mentioning that Mr Leedman has also chaired Neurotech International (ASX:NTI) which is developing the use of very low THC oral cannabis drug candidate NTI164 for children with autism. During his tenure, the share price had risen over 1,000%.
“This is a natural progression for me,” Brian Leedman said.
“Everything I’ve done is highly relevant to getting BlinkLab to this point and strengthens my belief that we have something truly special here.”
