Case study: Cretostimogene Grenadenorepvec (CG0070)
CG0070 is an oncolytic viral therapy drug developed by CG Oncology, specifically targeting bladder cancer. The drug leverages a modified adenovirus to selectively infect and kill cancer cells while sparing normal cells. This selectivity is achieved through the genetic engineering of the virus to exploit the tumor-specific transcription factor E2F, which is active in many cancer types but repressed in normal cells.
https://pubmed.ncbi.nlm.nih.gov/23088985/
The first ever phase 1 dose escalation trial of CG0070 in 2012 achieved a complete response (CR) rate of 48.6% in 35 BGC-unresponsive NMIBC patients. As seen below, the lowest dose level of CG0070 achieved the greatest CR rates, with excellent CR rates at the next two levels, and no activity at the 4th dose level. Efficacy clearly does not increase with dose in this OV therapy, and the dosing regimen for all remaining trials remained at 1 x 10^12. This was advantageous as there were few dose-related toxicities at this level.
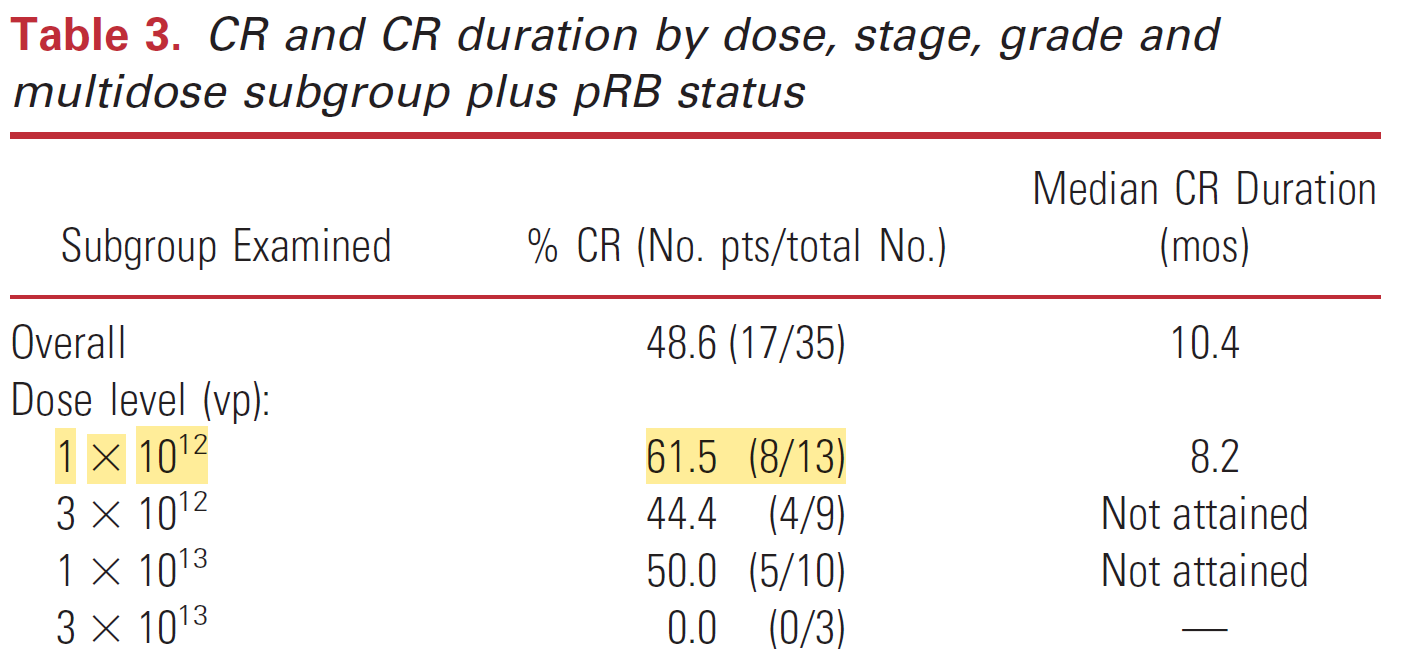
The trial included patients who had undergone at least one prior line of therapy, with an average of three previous treatments (ranging from 2 to 9). This consisted of a median of 2 BCG or BCG plus interferon treatments (range 1 to 9) and a median of 1 chemotherapy treatment (range 1 to 4).
CG0070 has demonstrated outstanding efficacy (>50% CR) in phase 2 trials alone and in combination with approved medicines (red border) and in a phase 3 trial (blue border).


Despite excellent efficacy in the original phase 1 dose escalation trial, successful phase two trials alone and in combination, a successful phase 3 trial with excellent efficacy, fast-track designation, and breakthrough therapy designation, CG0070 has not had commercial success over the 12-year span.
CG0070 achieved more CRs than CF33 has had SDs alone or in combination with Keytruda, and it has not become commercial in 12-years since the original phase 1 dose escalation trial.
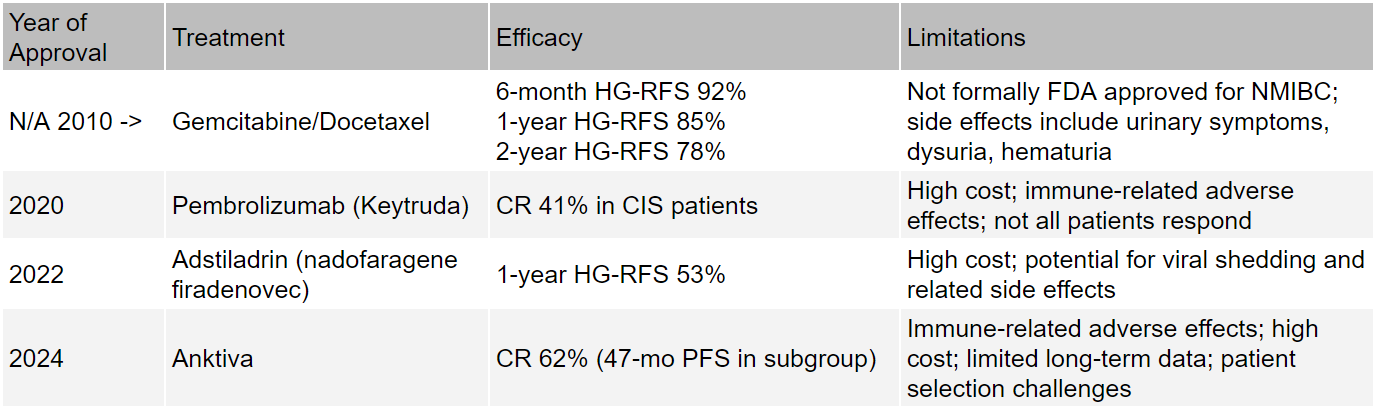
The reason CG0070 has not been effective is that there is enormous competition within BGC-unresponsive NMIBC. While radical cystectomy is the primary standard of care option for patients that do not respond to initial BGC treatment, the gemcitabine/docetaxel combination has been achieving excellent results since 2010. Data collected over a 10-year period demonstrated that the combination had a 6-month high-grade recurrence free survival (HG-RFS) of 92% which stays very high for up to 2-years (78%). Both gemcitabine and docetaxel are off patent exclusivity, meaning they are both cheap and effective options.
https://www.onclive.com/view/fda-grants-fast-track-breakthrough-therapy-designations-to-cretostimogene-grenadenorepvec-for-nmibc
https://www.onclive.com/view/fda-approves-nadofaragene-firadenovec-for-high-risk-non-muscle-invasive-bladder-cancer
https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer
https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-nogapendekin-alfa-inbakicept-pmln-bcg-unresponsive-non-muscle-invasive-bladder-cancer
https://www.urologytimes.com/view/intravesical-gemcitabine-docetaxel-for-nmibc-appears-safe-and-efficacious
This represents a very potent example of the tall task any therapy has when commercial success requires displacement of the market and taking patients away from established, cheaper, and effective competitors.
I think the focus on cholangiocarcinoma is a commercial disaster for IMU that no-one is talking about. NMIBC occurrence rates are 7x that of cholangiocarcinoma in the US. If CF33 can’t achieve at least 50% CR rates from a phase 2 dose in a specific set of cholangiocarcinoma patients, your path will be significantly less than that of CG0070. Even if it does, it’s probably still significantly less than CG0070 and investors are probably waiting a very long time (2036) to most likely not establish commercial success. Thus, clinical and commercial risk is very high.
 (20min delay)
(20min delay)









