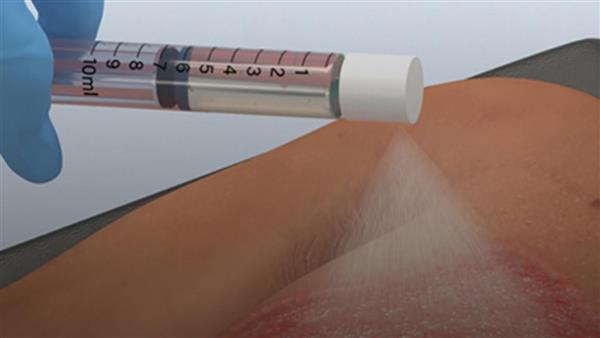
- AVITA Medical (AVH) gains FDA approval to launch its RECELL spray-on skin system to treat full-thickness skin defects
- This provides relief for a broad label of full-thickness skin defects, including degloving, surgical excision and skin cancer
- Currently, skin grafting is the standard of care for full-thickness skin defects, with multiple risks attached to the procedure
- The commercial launch is set to take place on July 1, 2023
- AVITA Medical is up by 10.7 per cent, trading at 4.75 at market close
AVITA Medical (AVH) gained FDA premarket approval for the use of its RECELL spray-on skin system to treat full-thickness skin defects.
AVITA’s Medical CEO Jim Corbett described the news as a “landmark approval,” representing an inflection point for the company.
“The FDA approval now offers surgeons a best-in-class treatment option for a multitude of severe wounds within inpatient and outpatient settings.”
The advancement offers relief for a broad label of full-thickness skin defects, such as wound injuries after traumatic avulsion, such as degloving, surgical excision, including necrotising soft tissue infection, or resection, like skin cancer, thereby dramatically expanding the company’s market opportunity at least five times.
“On behalf of AVITA Medical, I’d like to express my utmost appreciation to the many patients and healthcare providers who partnered with us to help bring our innovative technology to more patients across the U.S,” Mr Corbett said.
The AVITA sales team is prepared for the commercial launch, which will commence from July 1, 2023.
AVH was up 10.7 per cent, trading at 4.75 at market close.
