Argenica Therapeutics (ASX:AGN) announced on Monday the company has won rare pediatric disease designation (RPDD) for its flagship drug ARG-007.
Shares jumped 15.7% in the first hour of trade to 66cps.
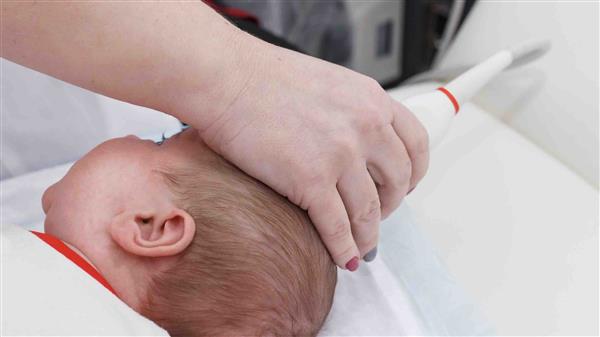
ARG-007 is the treatment Argenica is hoping to commercialise as a drug to improve outcomes in infants with brain tissue complications following brain injury.
In scientific parlance, the exact condition is called Hypoxic Ischaemic Encephalopathy (HIE). In layman’s terms: inflammation of the brain caused by lack of oxygen.
The RPDD designation ultimately allows Argenica something of a fast-track status through the US regulator’s hoops towards commercialisation.
“The granting of the RPDD provides one key
substantial benefit to Argenica,” the company wrote on Monday.
“Upon approval of a New Drug Application (NDA) for ARG-007 in HIE, the FDA may award a Priority Review Voucher (PRV) provided that HIE is the first indication for which the drug is approved.”
That voucher can be ‘redeemed’ to “accelerate the review of a subsequent marketing application.” Alternatively, the company can also sell or transfer that voucher to a third party.
The company noted on Monday that prices for such vouchers can range up to the tens of millions of dollars.
The value proposition is that Argenica implies it’s the only player with a drug to treat HIE.
“There are currently no therapeutic drugs available to treat this devastating condition,” Argenica chief Dr Liz Dallimore said.
“This RPDD will provide the Company with potential significant upside at the end of a clinical program in HIE to receive a priority review to get the drug on the market quickly.”
AGN shares were worth 66cps at 10.50am AEDT on Monday.
