That massive carbon footprint exists because although the Haber-Bosch process represents a huge technological advancement, it’s always been an energy-hungry one. The reaction, which runs at temperatures around 500 °C and at pressures up to about 20 MPa, sucks up about 1% of the world’s total energy production. It belched up to about 451 million t of CO2 in 2010, according to the Institute for Industrial Productivity. That total accounts for roughly 1% of global annual CO2 emissions, more than any other industrial chemical-making reaction (see page 23).
Strike Energy unveils $2.3b WA urea project
Robert Guy
Strike Energy has unveiled plans to develop a $2.3 billion ammonia and urea project near Geraldton in Western Australia, and will start marketing equity participation in the project later this year.
The company, which includes Future Fund director John Poynton and National COVID-19 Commission chairman Nev Power on its board, has completed a feasibility study on a 1.4 million tonnes a year urea production facility.
The project - named Project Haber -would be fed with gas from Strike's Perth Basin resources. Project Haber will consume 86 terajoules of gas a day.
Ammonia by the numbers
157.3 million:
Metric tons of NH3 produced worldwide in 2010
451 million:
Metric tons of CO2 emitted by NH3 synthesis worldwide in 2010.
~1%:
Percentage of global CO2 emissions that come from NH3 synthesis.
Sources: Institute for Industrial Productivity.
The carbon footprint of ammonia synthesis goes well beyond its energy demands. Hydrogen used for the reaction comes from natural gas, coal, or oil through processes that release CO2. According to a 2013 joint report from the International Energy Agency, the International Council of Chemical Associations, and the Society for Chemical Engineering and Biotechnology, CO2 emissions from hydrogen production account for more than half of those from the entire ammonia production process. In total, from hydrocarbon feedstocks to NH3 synthesis, every NH3 molecule generated releases one molecule of CO2 as a coproduct
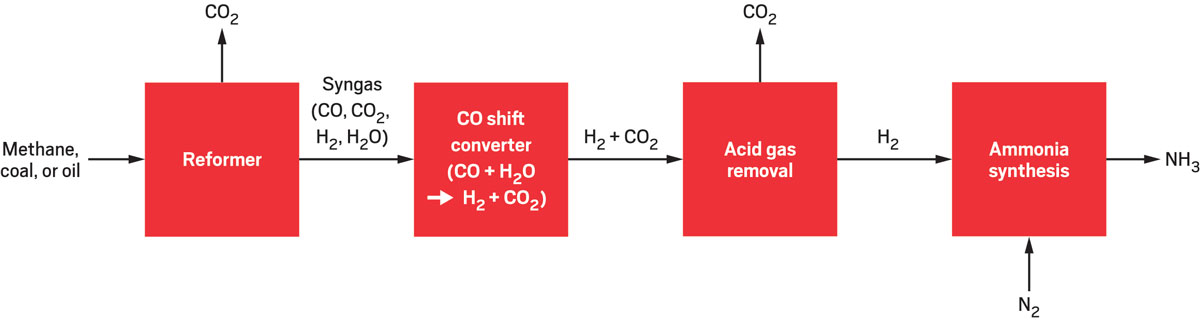
Current process
Today, ammonia synthesis starts with generating hydrogen gas from fossil-fuel feedstocks. A reformer turns the feedstocks into a mixture of gases called synthesis gas (syngas), which includes hydrogen. A CO shift converter combines water and the carbon monoxide from syngas to form CO2 and more hydrogen, and then acid gas removal isolates the hydrogen for ammonia synthesis. This process releases CO2 at various steps along the way.