Some reading to pass the time away.
taken from a FB post by ACW today.
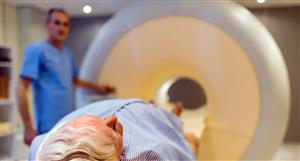
Biotech DailyFriday August 2, 2024Daily news on ASX-listed biotechnology companiesDr Boreham’s Crucible:Actinogen MedicalBy TIM BOREHAMASX code: ACWShare price: 7.1 cents; Shares on issue: 2,711,639,883; Market cap: $192.5 millionChief executive officer: Dr Steven GourlayBoard: Dr Geoff Brooke (chair), Dr Gourlay, Dr George Morstyn, Malcolm McComas, DrNicki VasquezFinancials (June quarter 2024): revenue $100,000, cash outflows $5.1 million, cashbalance $9.5 million, quarters of funding: 1.9, but says it has resources to late 2025.Identifiable major holders: Biotech Venture Fund 9.2%, Dr Steven Gourlay 3.6%An imminent trial result may validate whether Actinogen’s Xanamem is ‘the next Ozempic’.Originally developed for diabetes and embraced as a weight loss drug by the beautifulpeople, Ozempic is also showing promise in the $US4.8 billion-a-year Alzheimer’s diseasetreatment market.Actinogen chief Dr Steve Gourlay says type 2 diabetes is a known risk factor forAlzheimer’s and GLP-1 drugs have been shown to reduce the chance of developing thedisease in diabetics.Not surprisingly, drug companies are on the GLP-1 case, with Novo Nordisk and Eli Lillyboth carrying out large phase III oral formulation trials. A drug could be approved by asearly as 2027.Xanamem shares some similar traits to the mechanisms of GLP-1 drugs, in that itimproves insulin sensitivity and may share some of the same metabolic benefits in thebrain.However, it has been developed by Actinogen to target the toxic effects of the “stresshormone” cortisol in the brain - something that GLP-1 drugs don’t do.Xanamem also has a different safety profile.“GLP-1 drugs work by in part by preventing your stomach emptying, which makes you feelfull, with nausea as a main side effect,” Dr Gourlay says. “This is not a good profile formany Alzheimer’s patients who forget to eat and are already losing weight.”A bit of historyActinogen listed in October 2007 at 50 cents apiece, with an initial focus on soil-derivedantibiotic-like compounds called actinomycetes (hence the Actinogen name).In a radical course correction, Actinogen acquired Xanamem as UE2343 in 2014 fromEdinburgh University, which had completed a phase I trial.Australian clinical development started in 2015.Dr Bill Ketelbey joined the company as CEO in December 2014. Dr Ketelbey was involvedin developing Aricept, which remains the leading Alzheimer’s treatment despite beingdeveloped almost 30 years ago.Dr Gourlay succeeded Dr Ketelbey in early 2021. Dr Gourlay previously worked in seniorroles at Genentech and then with Dr Geoff Brooke (now Actinogen chair) at GBS VenturePartners.As founding chief medical officer of the San Francisco-based Principia Biopharma, hehelped to take two immunology programs to advanced trials, at which point Sanofiacquired the company for $US3.7 billion ($A5.5 billion).About XanamemXananem is a brain tissue cortisol synthesis inhibitor, potentially with applications forpsychiatric and neuro-degenerative diseases beyond Alzheimer’s and depression (suchas Fragile X syndrome and cognitive impairment in schizophrenia).Other Alzheimer’s drugs work by inhibiting the formation of amyloid proteins, which formas plaques and are thought to be a key contributor to the disease.Xanamem takes a different tack by inhibiting production of cortisol, which is synthesizedby an enzyme called 11 beta HSD1.Cortisol is a naturally occurring stress hormone and essential for the body, but elevatedlevels over a long period are thought to contribute to both Alzheimer’s and mild cognitiveimpairment (which can often lead to the former).Xanamem is expected not to interact with other drugs so could be used in older patientstaking medications for conditions such as cholesterol and blood pressure.To be effective, any drug first must cross the blood-brain barrier, the organ’s naturaldefence against foreign agents. Xanamem appears to do this.To date, Actinogen has studied 11 beta HSD1 inhibition in more than 350 patients andvolunteers.Learning from past mistakesActinogen has staged a remarkable recovery from the dark days of mid-2019, when itskey trial - Xanadu - failed.The study of 185-patients with clinical mild Alzheimer’s disease showed Xanamem over12 weeks worked no better than placebo.But the company cut the data another way - as you do - by examining the stored bloodsamples of 72 of the enrollees to see if they had ‘confirmed’ Alzheimer’s. This wasmeasured by elevated blood levels of a protein biomarker called pTau181 orphosphorylated tau.The results showed half the patients had a low level of the biomarker and showed noprogression at all - and thus possibly didn’t have Alzheimer’s disease in the first place.In patients with a high level of the biomarker, indicating ‘real’ Alzheimer’s, twice as manyXanamem-treated patients had stable or improved disease relative to placebo, with a 60to 80 percent reduction in disease progression over 12 weeks.On trial (1)The company is running two phase II trials, the first of which is about to report results.The phase IIa trial, Xanacidd, is studying the ability of Xanamem to improve cognitivedysfunction (difficulty thinking, remembering and solving problems) associated with majordepression.The trial has completed visits with 167 patients enrolled and will report top-line data on theprimary endpoint of cognition, with secondary endpoints including reducing depression.Over six weeks, the patients receive 10 milligrams of either Xanamem or a placebo daily(in some cases in addition to their existing anti-depressant drugs).The primary endpoint is a composite of three computerized Cogstate tests for workingmemory and attention. A key secondary endpoint is the commonly used Montgomery-Asberg Depression Rating Scale.Dr Gourlay says anti-depressants might improve mood, but they do little for the cognitiveimpairment or foggy thinking of patients with depression. “Demonstrating improvedcognition in patients with depression could pave the way for Xanamem to be used in otherpsychiatric conditions such as schizophrenia, where cognitive impairment is profound.”On trial (2)A second phase IIb trial, Xanamia, is recruiting patients with biomarker-positive mild tomoderate Alzheimer’s disease.The 220 patients are dosed over 36 weeks - also with 10mg - and are included if theyhave elevated p-Tau blood levels.The patients are assessed on both cognition and Alzheimer’s progression.“We believe we have already validated the target by showing improved cognition inhealthy older volunteers and a potentially a big clinical benefit in biomarker-positivepatients with Alzheimer’s,” Dr Gourlay says.Interim results - covering the first 100 patients at the 24-week mark - are expected in mid-2025. Final results are expected in mid-2026.What next?Dr Gourlay says getting a depression drug to market would require at least two morepivotal trials, about twice the size of the current trial.He expects the depression drug to be progressed with a partner, while the company wouldlike to expand the current Alzheimer’s study to more sites, off its own bat.“This potentially could form one of the pivotal studies,” he says. “We would start thesecond phase III pivotal study as soon as we could and hopefully that would bring forwardapproval by a year or so.”The company expects FDA breakthrough designation for Alzheimer’s and potentially forcognitive impairment in depression.Eyeing the competitive landscapePlenty of Alzheimer’s drug development is taking place but so far there is no magic bullet.In July, the FDA approved Eli Lilly’s Kisunla (donanemab), a monoclonal antibody infusionfor mild cognitive impairment or early Alzheimer’s that targets the amyloid protein.In February, sales of Biogen’s first amyloid antibody, Aduhelm (aducanumab), werediscontinued, reportedly because of poor sales and/or side effects.Biotech scholars will recall that the FDA in 2021 approved Aduhelm on the basis of onlyone positive phase III trial, snubbing the advice of its own 10-member expert committee.(Three of them quit, with one describing the decision as the worst drug approval inhistory).Dr Gourlay says such drugs have set a low bar for approval because of their modestbenefits and need for intensive safety monitoring and side effects.“The amyloid drugs have probably shown the best data they can, so we are unlikely to seea better story emerge with amyloid as the target.”Finances and performanceActinogen has completed a placement and rights offer that raised $8.9 million, at 2.5 centsapiece. Holders received one share for every 15 held, plus one option for every twoshares subscribed for.The options are exercisable at five cents within three years. The company also hasunlisted options exercisable at 3.75 cents, expiring in 2026 and if fully exercised all ofthese options would raise up to $16 million.Actinogen’s new CFO Will Souter says that with around $9.5 million in the bank and anexpected $8 million Federal Research and Development Tax Incentive, the company isfunded to late 2025.Current cash burn is “elevated” but is expected to subside in the December quarter withthe completion of the cognition/ depression trial.Unlike many other drug developers, Actinogen carries out most of the clinical work inhouse, rather than cede it to a contract research organization (CRO).“It is significantly cheaper than using a CRO and trial staff at sites love the directrelationship,” Dr Gourlay says.Over the last year Actinogen shares have traded between two cents (in a prolongedperiod between August 2023 and January 2024) and the current zenith. Historically theshares peaked shortly after listing in October 2007, at 55 cents and hit a nadir of one centin September 2019.Dr Boreham’s diagnosis:Globally, 55 million people have Alzheimer’s disease - 500,000 in Australia - and theWorld Health Organisation rates the disease as the seventh-biggest cause of death (as of2020).The global Alzheimer’s therapeutics market size is estimated to grow from $US4.82 billionin 2023 to around US$8.18 billion by 2032.If Xanamem succeeds, Edison Research estimates peak sales of $US5 billion in the early2030s, while depression is a $US2 billion market.Naturally, Actinogen has a long way to go, but Dr Gourlay is heartened by some big-tickettransactions in the neurology sector at pre-approval or even early stage.Last December, Bristol Myers Squibb acquired the Nasdaq-listed Karuna Therapeutics for$US14 billion. Karuna is developing its Karxt agent for schizophrenia and Alzheimer’s,having lodged an FDA submission for the former.Karxt works by reducing dopamine levels in the brain, so we there’s more than one way toskin this rabbit.As for the Ozempic-style drugs, do they pose a competitive threat to Actinogen in thesame manner as Resmed (sleep apnoea) and CSL’s Vifor arm (kidney dialysis)?Dr Gourlay’s premise is there will be a place for the ‘Ozempics’ in the Alzheimer’s space:“they probably will be better than the anti-amyloids but they won’t be the safe and effectiveoral treatment, such as the one we are developing”.So, the short answer is “no”.Ozempics or not, the Alzheimer’s treatment Olympics is still an open race to the winner’spodium.Disclosure: Dr Boreham is not a qualified medical practitioner and does notpossess a doctorate of any sort – or a gold medal.Biotech Daily can be contacted at: PO Box 5000, Carlton, Victoria, Australia, 3053
- Forums
- ASX - By Stock
- ACW
- Ann: ACW XanaCIDD depression trial results expected early August
ACW
actinogen medical limited
Add to My Watchlist
4.00%
 !
2.4¢
!
2.4¢
Ann: ACW XanaCIDD depression trial results expected early August, page-93
Featured News
Add to My Watchlist
What is My Watchlist?
A personalised tool to help users track selected stocks. Delivering real-time notifications on price updates, announcements, and performance stats on each to help make informed investment decisions.
 (20min delay) (20min delay)
|
|||||
|
Last
2.4¢ |
Change
-0.001(4.00%) |
Mkt cap ! $76.25M | |||
| Open | High | Low | Value | Volume |
| 2.5¢ | 2.5¢ | 2.4¢ | $8.444K | 350.1K |
Buyers (Bids)
| No. | Vol. | Price($) |
|---|---|---|
| 18 | 2914875 | 2.3¢ |
Sellers (Offers)
| Price($) | Vol. | No. |
|---|---|---|
| 2.4¢ | 52751 | 2 |
View Market Depth
| No. | Vol. | Price($) |
|---|---|---|
| 18 | 2914875 | 0.023 |
| 17 | 3564580 | 0.022 |
| 11 | 6819815 | 0.021 |
| 19 | 4329980 | 0.020 |
| 15 | 2338536 | 0.019 |
| Price($) | Vol. | No. |
|---|---|---|
| 0.024 | 52751 | 2 |
| 0.025 | 1863497 | 8 |
| 0.026 | 1081642 | 9 |
| 0.027 | 1774565 | 6 |
| 0.028 | 482142 | 5 |
| Last trade - 13.30pm 16/07/2025 (20 minute delay) ? |
Featured News
| ACW (ASX) Chart |
The Watchlist
WCE
WEST COAST SILVER LIMITED
Bruce Garlick, Executive Chairman
Bruce Garlick
Executive Chairman
Previous Video
Next Video
SPONSORED BY The Market Online