Hi Metro,
Fair call - your courteous request deserves a courteous reply - happy to oblige.
I'm sure we both accept that it's easy to put a positive or a negative spin on the facts whilst also being genuine in our thought process at the same time.
With this post I'll attempt to convince you of exactly why I remain interested in Isonea - I will give you the positive, the whole positive, and nothing but the positive.
___
Starting with the good gentleman I've been hardest on:
1) Do I believe Mr Michael Thomas has the right experience and credentials to increase the value of ISN securities and steer the company towards a successful future?
Imho, yes I believe he does
Mike Thomas
Mr. Thomas has over 22 years of experience in the healthcare industry in various executive positions. He is the President & CEO of Appian Partners, a healthcare advisory firm focused on strategy development and implementation for medical device and healthcare services firms ranging in size from early stage start up to Fortune 50 global conglomerates. Prior to that, he was President and CEO of Sleep Solutions, Inc., (SSI) a venture capital backed medical device manufacturer and national healthcare service provider. He raised over $50MM in venture capital funding to develop and launch a new industry in the $5 billion Sleep Medicine market. He successfully lobbied the largest Managed Care Organizations (MCOs) and the Centers for Medicare and Medicaid Services (CMS) to alter their medical policy and approve reimbursement for in home sleep apnea diagnostic testing services. Mr. Thomas was also a member of the Board of Directors for AdvaMed, the world's largest and most prestigious medical device association. While a BOD member for AdvaMed, he participated in the successful renegotiations with the FDA for the MEDUFMA II legislation (the FDA's regulatory approval process for medical devices), with leaders on Capitol Hill regarding the Remote Monitoring legislation, and sat on the BOD sub committee for International Affairs. Prior to SSI, he was Executive Vice President of Sales and Marketing for National Sleep Technologies Inc. (NST). As an executive and Board member, he identified and closed 12 acquisitions representing over 80 sleep labs that led to NST becoming the largest U.S. sleep-testing company at that time. He helped create the first national brand in the highly fragmented sleep apnea diagnostic lab testing services market. He recruited and managed a sales and marketing team that achieved a same-store sales growth rate that was 2 times greater than the industry average for four consecutive years. He helped raise over $15MM in venture capital financing and eventually sell the company to a wholly owned subsidiary of GE Medical (NYSE: GE). Previous to SSI, Mr. Thomas was instrumental as the Vice President of Sales in building sales and marketing for Patient InfoSystems, Inc., (NASDAQ: PATI) a healthcare I.T. start-up specializing in the development of disease management programs for the healthcare industry. During his tenure, he hired, trained and managed a national sales force and was instrumental in taking the company public within two years of its formation. Having held various sales and marketing positions, including seven years with Merck and Glaxo Wellcome, he has earned numerous awards for sales and management achievements. Mr. Thomas graduated from Cornell University with a bachelor's degree in Microbiology.
Who are Appian Partners
"Seasoned executives with nearly three centuries of combined expertise are available to help you grow your company."
Services
•Angel, Venture Capital, Private Equity fundraising ?Business plan creation
?Seed funding
?Product development and launch funding
?Growth funding
?Mezzanine funding
•Primary market research
•Industry / key stakeholder roundtable symposia ?Organize
?Moderate
•Reimbursement strategy development and implementation ?Medicare (CMS)
?Manage Care Organizations / Third party payors
•Regulatory strategy creation and oversight ?Clinical trial protocol development
?FDA 510k and PMA
•Mergers & Acquisition planning and execution
•Business development and strategic partnership identification, negotiation, implementation, and ongoing relationship management
•Executive coaching for C-suite managers
•Build and execute sales and marketing strategies
Appian Partners
___
2) With a sp of $0.005c - Mkt Cap of $11,651,280 and the forward looking strategy as set out by the new management, do I believe the potential upside may present itself in the short, medium or long term?
Yes, I do - Absolutely!
Business Strategy
iSonea is laying the groundwork for the successful commercialization of its ARM product line by securing worldwide regulatory and reimbursement clearances and building an extensive network of distributor partners across the United States, Europe and Asia Pacific. The Company is initially targeting the world’s major developed markets, which include the U.S. (40% of the world total) and Europe (30% of the total). To support the U.S. rollout, iSonea is establishing sales and clinical support operations in southern California and making ARM devices available to pulmonary and pediatric respiratory specialists to build clinical experience. In Europe, the Company is initiating product sales through a network of respiratory product distributors serving the UK, Germany and other major markets, with iSonea providing pre-sale and post-sale support.
The Company has also signed distribution agreements with partners in Australia and India and is seeking regulatory approval for its ARM devices in Japan. Already the world’s 2nd largest respiratory market, Japan is experiencing an increasing incidence of COPD and other respiratory conditions.
For the U.S. market, iSonea is implementing a multi pronged marketing strategy that will push the devices to physicians and create medical demand. The Company is partnering with distributors already serving the respiratory care market, undertaking clinical studies that validate ARM technology as equal to or better than the existing standard of care, laying the groundwork for insurance reimbursement, establishing data portals that connect patients with their physicians and promoting ARM products directly to the medical community.
The cornerstone of the Company’s consumer strategy is developing a personal wheeze monitor and wheeze monitor with smart phone applications that can be sold over-the-counter. iSonea plans to drive consumer demand through smart phone monitoring tools and applications in asthma, a social media campaign that will educate consumers about ARM technology and build brand awareness and re engineering its technology platform for lower COGs and average selling prices.
Some of the key steps in the roll-out include the following:
Enlist support of Key Opinion Leaders: Since iSonea is introducing a new paradigm for asthma monitoring, support from key opinion leaders in the medical community is needed to build broad acceptance for the technology.
The prescriber audience that the Company must reach in the U.S. includes some 320,000 pulmonary specialists and general practice physicians, thousands of hospitals and testing facilities and 25 million asthma patients.
To build credibility with opinion leaders, iSonea is expanding clinical studies validating ARM technology. The Company has already completed multi-center clinical studies of its technology in leading U.S. and Australian medical centers and PFT labs in the U.S., Israel, Australia and Europe. Medical institutions that have been involved in ARM studies include Royal Melbourne Hospital (Australia), the Bromton Hospital (UK), Mass General Hospital (USA) and Oakland Children’s Hospital (USA).
Researchers have detailed the successful results of these studies in scientific papers that have been submitted for publication in prominent respiratory and pulmonary medical society journals. These studies not only confirm the value proposition of ARM devices for asthma monitoring, but set the stage for exploring applications in other respiratory conditions such as Chronic Obstructive Pulmonary Disease (COPD), persistent cough and cystic fibrosis.
iSonea has introduced its technology at the conferences of the American Thoracic Society, American College of Chest Physicians, European Respiratory Society, American Academy of Respiratory Care, American Academy of Asthma, Allergy and Immunology, Medica and the Australian Respiratory Society. An article describing the Company’s cough validation methods and results recently appeared in Cough Journal and scientists have delivered keynote lectures about ARM technology at national scientific academy and society meetings.
Secure third party reimbursement: Securing third party reimbursement is critical for building a sustainable business model among physician prescribers in the U.S. iSonea technology has already been assigned a temporary reimbursement code (CPT III) by the American Medical Association, which has been given the task of assigning CPT approval codes to medical devices, services and tests. Securing a CPT code is necessary before product sales can begin. A CPT III code means Medicare will track the use of an emerging technology, which must happen before the American Medical Association can recommend reimbursement and approve pricing.
iSonea plans to use data from its expanded clinical study to support an upgrade in the CPT code from Category III to Category I, which would allow physicians to prescribe the technology and be reimbursed by insurance payers. The study data will also be used to solidify reimbursement in the EU countries, Japan, India and China.
Reimbursement should cover physician-prescribed device sales and diagnostic services such as sleep studies. At present, reimbursement varies by payer. In some instances, iSonea has been able to obtain up to 40% reimbursement from private insurers, which is a much higher rate than most new medical devices collect.
When the Company secures a Category I CPT code, reimbursement will increase significantly. Comparable diagnostic services are reimbursed between $60 and $250 in some developed markets.
The rollout of personal wheeze monitoring devices that can be marketed directly to consumers over-the-counter will accelerate market uptake of ARM technology and allow iSonea to quickly build brand awareness and market share among patients and their physicians.
Build global distribution network: iSonea is establishing a global network of distribution partners anchored by large companies like Omron, and including regional and national companies that can introduce ARM technology to targeted physicians in key markets. The Company has signed agreements with 23 distributors worldwide to-date and is utilizing those channels to conduct pilot studies so that physicians can gain experience with the devices and software.
The Company’s decision to sell through distributors rather than a direct sales force makes good business sense since iSonea needs to preserve cash and allocate available resources for the expanded clinical study. Its relationships with regional firms and large multinational companies should allow iSonea to reach its targeted audience quickly and costeffectively.
Potential customers for ARM technology include tens of thousands of PTF labs, sleep centers and hospitals, 20,000 pulmonary and allergy physician specialists, 58,000 pediatricians and 250,000 primary care and emergency care physicians.
Develop mobile asthma monitoring device with smart phone applications. iSonea is applying its core strengths in ARM technology, software and algorithms to the mobile health platform by developing health management applications and digital monitoring hardware that can be integrated with smart phones. The Company’s next generation smart phone device (Mobi-ARM ™) makes asthma monitoring possible for anyone with a smart phone. This more convenient method for continuous asthma monitoring will enable patients and physicians to effortlessly adhere to global asthma management guidelines that have been recommended by specialists, but are rarely followed in practice.
Mobi-ARM will be unique to iSonea – no direct competition in smart phone enabled acoustic respiratory wheeze monitoring exists. As a result, iSonea should be ideally positioned as the first mover in a potentially huge market for mobile asthma monitoring. The market for smart phones already exceeds billions of dollars and consumer demand for health management applications for these devices is skyrocketing. According to Pew Research Center, the number of health management applications for smart phones has
nearly tripled since last year approximately 3,000 to nearly 9,000 today and Pew forecasts 50% growth and more than 13,000 applications by next July. A rising installed base created demand for more apps. There were 500 million smart phones sold globally in 2011 and unit sales are projected to exceed one billion phones sold annually by 2015.
Penetrations rates for health management applications for smart phones are especially high in the U.S. where nearly 1/3rd of all adults downloaded a health management app this year. Consumer appetite for health management applications and mobile monitoring creates a receptive audience for the roll out of Mobi-ARM in the U.S.
Isonea
___
3) Do I believe wireless technologies are going to completely transform health care?
Without a single doubt in my mind, yes I do!
Wireless Health Care
Wireless technologies are about to transform health care, and not a moment too soon
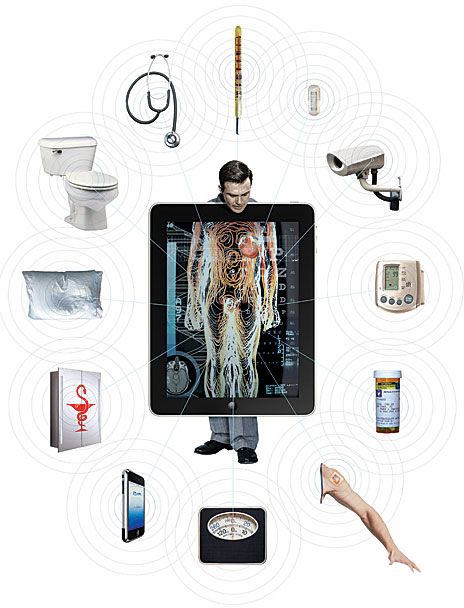
Imagine a world in which your medicine cabinet notices that you are due for a prescription refill and calls it in. A sensor implanted under your skin detects a fluid buildup in your lungs and alerts your doctor, who decides your heart medication needs an adjustment and contacts the pharmacist to change your dosage. Meanwhile, sensors in your toilet confirm that your body has adjusted well to your other medications but sees indications that you may be a borderline diabetic. Your doctor, given these readings and your family medical history, suggests that you change your diet. Noting that fact, your bathroom scale asks you to punch in a weight-loss goal and starts giving you a regular progress update. Your medical checkup isn't an annual event—it happens every day, simply as you go about your daily life.
If such ambient monitoring and intervention strikes you as a little creepy, think of it this way: It could avert a heart attack, stroke, or other medical crisis. It could keep you out of the hospital and save money for both you and the health care system. Part of the savings would come from radical changes in the management of chronic diseases, which in the United States eats up 75 percent of health care spending, or about US $1.9 trillion each year.
And a health-monitoring bathroom is not science fiction. This is what health care could look like within the decade, at least for some. Perhaps predicting such dramatic change within 10 years is overly optimistic. But the necessary technologies already exist or are close at hand, the need to reduce health care costs is real, and the current health care system demands change. What's more, a groundswell of support for wireless health care is rising from a diverse group of people and organizations. These include communities of consumers who want better ways to manage and measure their own health, like the Quantified Self movement and Health 2.0, which sponsors competitions to spur the development of new health care apps and devices. Also on board are nonprofit organizations like the West Wireless Health Institute, in La Jolla, Calif., where I work as chief medical and science officer. We focus on lowering health care costs through health technology innovation. The nonprofit LeadingAge Center for Aging Services Technologies is working to improve quality of life for the aging. For the third year in a row, the U.S. National Institutes of Health is hosting the global mHealth Summit, a conference examining the impact of mobile technologies on health care delivery, research, business, and policy. And hundreds of start-ups are exploring near-term and long-term ways to reform the delivery of health care.
Change is happening. Yes, a technological revolution in health care has been predicted before, but we are at an inflection point now, where wireless connectivity, personal cellular devices, pervasive sensing technologies, social networks, and data analytics are mature enough to make wireless medicine a reality. And there is a will as never before to find a way to reduce crippling health care costs. Already, new devices allow diseases like diabetes and chronic heart failure to be closely monitored outside the doctor's office; tools for tracking chronic kidney disease and a variety of lung disorders are sure to follow. Eventually, most health care will occur not during occasional visits to doctors' offices, clinics, or hospitals but continuously, during ordinary activities in people's homes, cars, and workplaces.
ieee spectrum
Say “hello” to the future of wireless health
The 2net™ Platform* from Qualcomm Life is truly novel, offering a set of wireless health solutions that can elegantly and reliably capture and deliver medical device data to integrated portals or databases from nearly ANY customers’ or collaborators’ wireless medical device for storage in a system designed for security. It’s a whole new way of connecting devices and liberating biometric data so that it becomes ubiquitous across the continuum of care.
The 2net Platform is a cloud-based system designed to be universally-interoperable with different medical devices and applications, enabling end-to-end wireless connectivity while allowing medical device users and their physicians or caregivers to easily access biometric data. With two-way connection capabilities and a broad spectrum of connection options, the 2net Platform will change the way you do business.
The 2net Platform supports SSL secure communication of data and is FDA listed as a Class I Medical Device Data System (MDDS). As an MDDS, it is designed, developed and manufactured in accordance with a quality system compliant with ISO13485 standards, meaning it aligns with the quality requirements of U.S. and international regulatory agencies in the healthcare industry.
There are four gateways onto the 2net Platform’s data center:
1.A standalone FDA-listed external device -- the 2net Hub*
2.Medical devices with an embedded cellular component
3.Medical data sent from mobile phones
4.Service platform integration between the medical grade 2net Platform to customer and collaborator service platforms using application programming interfaces (APIs)
We designed the cloud-based 2net Platform to provide a high degree of security and reliability to seamlessly store and transfer data once it has been acquired from the medical device so it can be shared with the appropriate audiences, which could include designated health care service companies, providers, payors, pharmaceutical companies, and application and device collaborators. The biometric data is stored in the 2net Platform’s Payment Card Industry, or PCI-compliant, data center. The data is encrypted in motion and at rest, and transmitted to the manufacturers’ interface of choice for the end-user.
The 2net Platform enables a single place where near real-time data across a wide range of device types, makes and use cases, can be accessed, unlocking and potentially improving health care delivery. To learn more about the 2net Platform, please download the 2net Platform white paper, and for an overview of how the 2net Platform works, please download the 2net Platform spec sheet.
The 2net Hub*
The 2net Hub, one of the four gateways used to access the 2net Platform’s data center, delivers a new dimension of short-range radio flexibility, security and seamless data transfer, while serving as the information highway for machine-to-machine (M2M) connectivity for medical devices into and out of the home. The 2net Hub is an FDA-listed, compact “plug-and-play” gateway that supports Bluetooth, Bluetooth Low Energy, WiFi, and ANT+ local area radio protocols.
The 2net Hub is Continua-certified, and supports 3G and 2G cellular communications. The 2net Hub supports SLL secure communication of data and is FDA-listed as a Medical Device Data System (MDDS). As an MDDS device, it is designed, developed and manufactured in accordance with a quality system compliant with ISO13485 standards, meaning it aligns with the quality requirements of U.S. and international regulatory agencies in the healthcare industry.
Introducing the 2net™ Platform
Add to My Watchlist
What is My Watchlist?