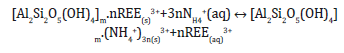Processing
Mineralogy is a key variable in determining the ease or difficulty, the cost of processing and extraction of REEs. Primary, hard rock deposits with the most abundant REE minerals such as bastnäsite, monazite, xenotime, loparite, parasite, and perhaps apatite is more likely to be economical compared with those deposits with eudialyte, allanite, or zircon [11,12]. Cause the latter group are more refractory and economic processing of REE from these minerals is not currently possible. In ion-adsorption ores, REEs are mostly present in ion-exchangeable phase of weathered granites, therefor, blasting, crushing, grinding, or mineral processing are not needed. REEs are easily extracted by ion exchange using diluted electrolyte solutions such as ammonium sulfate solution at ambient temperature [13-17].
There are different types of leaching methods have been utilized to extract REEs from ion-adsorption clays, such as heap, tank/pool, and in-situ leaching methods [18]. Previously, heap leaching was a commonly operated method which involves excavating minerals, placing them in a mound, and spraying them with solutions [17]. Tank/pool leaching involves placing the minerals into a tank/pool and immersed with solutions [18]. In-situ leach mining is now the dominating technology, given that there is less topsoil removed, the process can be performed on site, and the environmental impacts are reduced [19,20].
The schematic monovalent salt leaching extraction of REE3+ (Figure 1) suggested by recent studies [21] is:
In most cases, the rare earth leach liquor contains a significant amount of some impurities such as (NH4)2SO4, Al3+, Fe3+ and Ca2+, accompanied by a small amount of Fe2+, Pb2+ and Mn2+ [22]. The traditional process of extracting rare earth from such leach liquor is usually treated with chemical precipitation with oxalic acid or ammonium carbonate as precipitant, forming rare earth oxalate or carbonate, followed by washing and filtering and calcining into rare earth oxides [23,24]. However, the rare earth oxides need to be dissolved with hydrochloric acid before being separated by solvent extraction or used as materials for many rare earth users [25]. Although, they have been applied to industry practice, these precipitation technologies are complicated, time-and reagentconsuming, and the serious disadvantage is that these impurity ions cannot be removed, and the product purity is low, in addition, the rare earth yield is also low [26]. Recently, efficient nonprecipitation techniques such as solvent extraction, ion exchange, and liquid membranes has been developed and applied in some mining sites [27].
- Forums
- ASX - By Stock
- MEK
- Ann: High-Grade Rare Earths Continue at Circle Valley
Ann: High-Grade Rare Earths Continue at Circle Valley, page-8
-
-
- There are more pages in this discussion • 29 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
Add MEK (ASX) to my watchlist
 (20min delay) (20min delay)
|
|||||
|
Last
7.0¢ |
Change
-0.017(19.5%) |
Mkt cap ! $135.4M | |||
| Open | High | Low | Value | Volume |
| 7.5¢ | 7.5¢ | 6.9¢ | $3.941M | 54.38M |
Buyers (Bids)
| No. | Vol. | Price($) |
|---|---|---|
| 15 | 1947076 | 7.0¢ |
Sellers (Offers)
| Price($) | Vol. | No. |
|---|---|---|
| 7.1¢ | 796764 | 4 |
View Market Depth
| No. | Vol. | Price($) |
|---|---|---|
| 15 | 1947076 | 0.070 |
| 14 | 1831791 | 0.069 |
| 11 | 2149705 | 0.068 |
| 1 | 1500000 | 0.067 |
| 2 | 1000000 | 0.066 |
| Price($) | Vol. | No. |
|---|---|---|
| 0.071 | 796764 | 4 |
| 0.072 | 650481 | 4 |
| 0.073 | 130000 | 3 |
| 0.074 | 190000 | 2 |
| 0.075 | 340422 | 3 |
| Last trade - 16.10pm 04/11/2024 (20 minute delay) ? |
Featured News
| MEK (ASX) Chart |