My bad it is 20 years with five years patent exclusivity for a new drug.
But there is more to it than that, there can also be exclusivity added in some circumstances, some start with the patent and are concurrent and others can add to it.
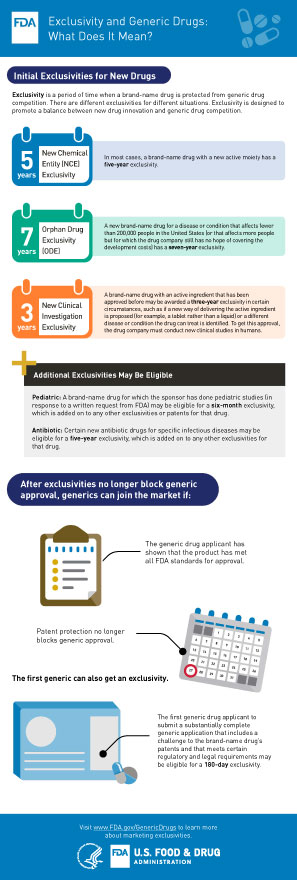
Exclusivity and Generic Drugs: What Does It Mean? Infographic (PDF - 470KB)
 Exclusivity
Exclusivity
- What is the difference between patents and exclusivity?
- How long is a patent term?
- How long does an exclusivity period last?
- Why does the exclusivity expire before the patent?
Patent before exclusivity?
Why does a particular drug product only have patents?
Only have exclusivity?
Have neither?
- What information related to pediatric exclusivity is listed in the Orange Book?
- Where can I find patent and exclusivity regulations in the Code of Federal Regulations (C.F.R.)?
- How is an NDA holder notified if their application has received a period of exclusivity?
Patents
- When should an NDA holder submit patent information?
- What is a patent submission date?
- Why doesn’t the Orange Book include patent submission dates for most records?
- How can an NDA holder request a patent submission date error correction?
- How should an NDA holder correct or request removal of patent information?
- Should an NDA holder submit patent information when seeking approval of a supplement?
- When may an NDA holder amend the description of the approved method(s) of use claimed by the patent?
- What actions must a pending ANDA or 505(b)(2) applicant take if patent information is untimely filed?
- Is there a specific format in which patent information needs to be submitted to the agency?
- To which submissions does the final rule apply?
- Does previously submitted patent information have to be re-submitted on the new Forms FDA 3542 and 3542a?
- Who do I contact with specific questions regarding what patents are eligible for listing in the Orange Book?
Orange Book Frequently Asked Questions
1. What is the difference between patents and exclusivity?
Patents and exclusivity work in a similar fashion but are distinct from one another and governed by different statutes. Patents are a property right granted by the United States Patent and Trademark Office anytime during the development of a drug and can encompass a wide range of claims. Exclusivity refers to certain delays and prohibitions on approval of competitor drugs available under the statute that attach upon approval of a drug or of certain supplements. A new drug application (NDA) or abbreviated new drug application (ANDA) holder is eligible for exclusivity if statutory requirements are met. See 21 C.F.R. 314.108, 316.31, 316.34 and sections 505A, 505E, and 505(j)(5)(B)(iv) of the FD&C Act. Periods of exclusivity and patent terms may or may not run concurrently. Exclusivity was designed to promote a balance between new drug innovation and greater public access to drugs that result from generic drug competition.
Back to Top
mce-anchor2. How long is a patent term?
Patent terms are set by statute. Currently, the term of a new patent is 20 years from the date on which the application for the patent was filed in the United States. Many other factors can affect the duration of a patent.
Back to Top
mce-anchor
3. How long does an exclusivity period last?
It depends on what type of exclusivity is at issue.
- Orphan Drug Exclusivity (ODE) – 7 years
- New Chemical Entity Exclusivity (NCE) – 5 years
- Generating Antibiotic Incentives Now (GAIN) Exclusivity– 5 years added to certain exclusivities
- New Clinical Investigation Exclusivity – 3 years
- Pediatric Exclusivity (PED) – 6 months added to existing Patents/Exclusivity
- Patent Challenge (PC) – 180 days (this exclusivity is for ANDAs only)
- Competitive Generic Therapy (CGT) - 180 days (this exclusivity is for ANDAs only)
See 21 C.F.R. 314.108, 316.31, 316.34 and sections 505A, 505E, 505(j)(5)(B)(iv), and Section 505(j)(5)(B)(v) of the FD&C Act.
Back to Top
mce-anchor4. Why does the exclusivity expire before the patent?
Patent before exclusivity?
Why does a particular drug product only have patents?
Only have exclusivity?
Have neither?
Patents and exclusivity apply to drugs in different ways. Patents can be issued or expire at any time regardless of the drug’s approval status. Exclusivity attaches upon approval of a drug product if the statutory requirements are met. Some drugs have both patent and exclusivity protection while others have just one or neither. Patents and exclusivity may or may not run concurrently and may or may not cover the same aspects of the drug product. Patents and exclusivities that have expired are removed from the Orange Book.
Back to Top
mce-anchor
5. What information related to pediatric exclusivity is listed in the Orange Book?
When pediatric exclusivity is obtained, a 6-month period of exclusivity is added to all existing patents and exclusivity on all applications held by the sponsor for that active moiety. Pediatric exclusivity does not stand alone, but attaches to existing exclusivity. When pediatric exclusivity attaches, in the patent column of the Orange Book, the patent is shown twice—once with the original patent expiration date and a second time reflecting the six month period of pediatric exclusivity linked to that particular patent. Related information can be found on the web page
Qualifying for Pediatric Exclusivity Under Section 505A of the Federal Food, Drug, and Cosmetic Act: Frequently Asked Questions on Pediatric Exclusivity (505A), The Pediatric "Rule," and their Interaction 



