Another important recruitment milestone, especially relevant during Covid-19 pandemic.
Physicians must be happy with outcomes for their patients on efti/keytruda combo - hence recruitment status.
Keytruda in combo with chemo is now generally accepted as the 'standard of care' for NSCLC. 1st line and
However, its approved only for patients expressing high PD-L1 ( >50%) = around only 30% of NSCLC patients.
ORR 48%. PFS 9 months
"Unmet need:•Modest efficacy of anti PD-1/PD-L1 for pts with <50% PD-L1 (~70% of total population)•Toxicity for patients / costs for health care systems of doublet chemo + PD-1/PD-L1 tremendous"
TACTI-002 is an all comer PD-L1 status and results thus far- ORR 53% and PFS could be over 12 months (ESMO Sept data update to confirm).
NB - this cohort NSCLC is 'second line' - which means patients are refactory (resistant or becoming resistant to PD1/PD-L1)
Therefore efti may be able to assist efficacy of keytruda (pembro) in second line scenario - with or without other immino combo?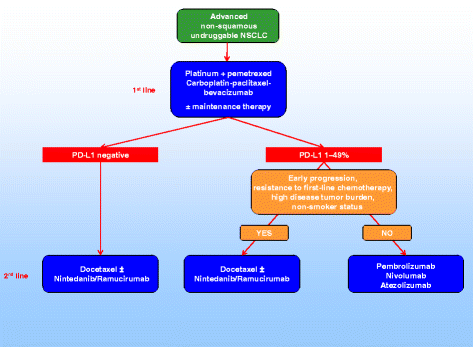
- Forums
- ASX - By Stock
- IMM
- Ann: Recruitment of stage 1 of part B in TACTI-002 study complete
Ann: Recruitment of stage 1 of part B in TACTI-002 study complete, page-7
-
- There are more pages in this discussion • 17 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
Add IMM (ASX) to my watchlist
 (20min delay) (20min delay)
|
|||||
|
Last
29.5¢ |
Change
0.015(5.36%) |
Mkt cap ! $428.5M | |||
| Open | High | Low | Value | Volume |
| 28.5¢ | 30.5¢ | 28.0¢ | $833.4K | 2.824M |
Buyers (Bids)
| No. | Vol. | Price($) |
|---|---|---|
| 8 | 388130 | 29.5¢ |
Sellers (Offers)
| Price($) | Vol. | No. |
|---|---|---|
| 30.0¢ | 39940 | 3 |
View Market Depth
| No. | Vol. | Price($) |
|---|---|---|
| 8 | 388130 | 0.295 |
| 8 | 134112 | 0.290 |
| 8 | 159211 | 0.285 |
| 15 | 235396 | 0.280 |
| 7 | 214272 | 0.275 |
| Price($) | Vol. | No. |
|---|---|---|
| 0.300 | 39940 | 3 |
| 0.305 | 116791 | 7 |
| 0.310 | 295779 | 7 |
| 0.315 | 160007 | 6 |
| 0.320 | 130000 | 3 |
| Last trade - 16.10pm 05/07/2024 (20 minute delay) ? |
Featured News
| IMM (ASX) Chart |









