Patients
|
Careers
|
Contact Us
About Isis
Antisense Technology
Drugs in Development
Investors & Media
News & Events
Press Releases
Archived Webcasts
Event Calendar
Corporate Governance
Stock Information
Financials
Shareholder Services
Strategic Alliances
E-mail this page Printer Friendly Version
Press Release
<< Back
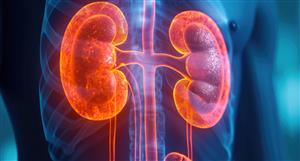
Genzyme and Isis Announce Submission of U.S. NDA for KYNAMRO™ (mipomersen sodium) in Homozygous Familial Hypercholesterolemia
Isis Eligible to Receive $25 Million Milestone Payment from Genzyme
CAMBRIDGE, Mass. & CARLSBAD, Calif.--(BUSINESS WIRE)--Genzyme, a Sanofi company (EURONEXT: SAN and NYSE: SNY), and Isis Pharmaceuticals Inc. (NASDAQ: ISIS) today announced that Genzyme has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking approval for KYNAMRO™ (mipomersen sodium) for the treatment of patients with homozygous familial hypercholesterolemia (HoFH).
“HoFH patients have aggressive, life-threatening cardiovascular disease starting at birth because of genetic mutations which severely impair LDL clearance and also trigger an overproduction of all atherogenic lipoproteins,” said Christie M. Ballantyne, M.D., Chief of the Sections of Cardiology and Cardiovascular Research, and Professor of Medicine and Genetics, Baylor College of Medicine. “Currently available therapies work on clearance whereas KYNAMRO™ uniquely targets Apo B production and production of all Apo B-containing, atherogenic lipoproteins, including VLDL, LDL, and Lp(a). Current options reserved for these patients are mechanical procedures such as LDL-aphereis or liver transplant.”
“This submission marks the second of two key milestones for the KYNAMRO™ program including the submission of the EU marketing authorization application last year.” said Vice President and General Manager of Genzyme’s Cardiovascular Business, Paula Soteropoulos. “These are important steps toward our goal of bringing an innovative solution to patients in great need of a novel, targeted therapy.”
The FDA submission for KYNAMRO™ is supported by the largest clinical trial conducted to date in the HoFH patient population. In the randomized, double-blind, placebo controlled, multi-center trial, significant reductions were observed in all atherogenic lipoproteins evaluated (including LDL-C, Apo B and Lp(a)) for patients receiving KYNAMRO™ who are already receiving a regimen of maximally tolerated lipid-lowering therapies including statins. Three patients (12 percent) treated with KYNAMRO™ withdrew due to adverse events. Consistent with other studies evaluating KYNAMRO™, commonly observed adverse events included mild to moderate injection site reactions and flu-like symptoms, as well as elevations in liver transaminases.
Isis will receive a $25 million milestone payment from Genzyme following FDA acceptance of the NDA submission. Provided the necessary approvals are granted, mipomersen would be marketed under the brand name KYNAMRO™, the name that has been submitted to health authorities for the investigational agent. The FDA has granted mipomersen Orphan Drug designation for the treatment of patients with HoFH.
“The last twelve months have been very successful for the KYNAMRO™ program,” said B. Lynne Parshall, J.D., Chief Operating Officer, Chief Financial Officer and Secretary of Isis. “We look forward to working with the U.S. and EU regulatory authorities to bring this treatment to patients in need.
There it is...yahooo! Validation for ANP
- Forums
- ASX - By Stock
- PER
- ann
ann, page-5
-
- There are more pages in this discussion • 32 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
Add PER (ASX) to my watchlist
 (20min delay) (20min delay)
|
|||||
|
Last
7.9¢ |
Change
-0.002(2.47%) |
Mkt cap ! $81.90M | |||
| Open | High | Low | Value | Volume |
| 8.0¢ | 8.0¢ | 7.9¢ | $230.1K | 2.904M |
Buyers (Bids)
| No. | Vol. | Price($) |
|---|---|---|
| 1 | 51000 | 7.9¢ |
Sellers (Offers)
| Price($) | Vol. | No. |
|---|---|---|
| 8.0¢ | 163520 | 2 |
View Market Depth
| No. | Vol. | Price($) |
|---|---|---|
| 1 | 51000 | 0.079 |
| 18 | 1244001 | 0.078 |
| 10 | 1037506 | 0.077 |
| 9 | 894271 | 0.076 |
| 10 | 1100649 | 0.075 |
| Price($) | Vol. | No. |
|---|---|---|
| 0.080 | 163520 | 2 |
| 0.081 | 344626 | 2 |
| 0.082 | 150000 | 1 |
| 0.083 | 368750 | 3 |
| 0.084 | 200000 | 1 |
| Last trade - 15.57pm 01/11/2024 (20 minute delay) ? |
Featured News
| PER (ASX) Chart |