For Balance view and search for the "tuth", keeping in mind that Covid-19 is suspected capable of quick mutation and vaccines may never be 100% effective and do not aid in the repair damage of humane tissue.
Pre-clinical COVID-19 vaccine trials begin at CSIRO
By Kashmi Ranasinghe
3 April 2020
3 minute read
We’ll be testing two vaccine candidates at our Australian Centre for Disease Preparedness (ACDP) formerly the Australian Animal Health Laboratory (AAHL) in Geelong, Victoria.
After months of research, we have another major update on our COVID-19 work. We have started pre-clinical trials for two vaccine candidates. This is happening at our Australian Centre for Disease Preparedness (ACDP) formerly the Australian Animal Health Laboratory (AAHL) in Geelong, Victoria.
The work — in which we play a critical role —— involves deep collaboration within Australia and overseas and forms part of a global rapid response to this pandemic.
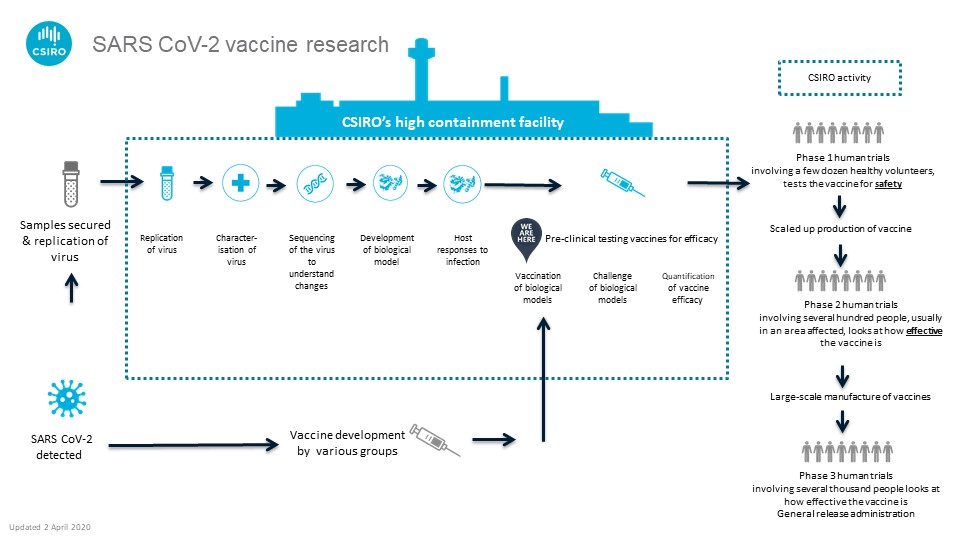
Our process for testing the new vaccine candidates
Pre-clinical trials to get the vaccine faster
Vaccines generally take 10-15 years to develop and test. But researchers like us are hoping to streamline this process. We’re working as part of a global alliance with the Coalition for Epidemic Preparedness Innovations (CEPI). The goal is to speed up traditional vaccine development and have one ready within 12-18 months.
CEPI asked us in January to start getting a better understanding of COVID-19 while the outbreak was in its early stages. Since then, and in consultation with the World Health Organization, CEPI has identified two candidates. These were based on readiness for testing.
The two vaccine candidates come from the University of Oxford in the UK and Inovio Pharmaceuticals in the US.
Both vaccines will undergo their first pre-clinical trials in Australia. We will infect ferrets with the vaccine. Then, we’ll leave them alone for four weeks. This gives the vaccine time to help the ferret’s immune system develop the right immune blockers for the virus.
After those four weeks, we’ll give them a dose of COVID-19. We’ll test the infection rate of the virus and the host’s immunity, telling us the vaccine’s effectiveness.
Our scientists will also test these vaccine candidates to figure out the best way for them to be administered. For example, it may be the classic intra-muscular injection we usually associate with vaccines. Or it could be a nasal spray which could provide us with better protection.
What else is happening at CSIRO?
We’re not directly involved in developing vaccine candidates for this virus. Instead, our research is focused on understanding the characteristics of COVID-19. After all, we were the first research organisation outside of China to generate enough stocks of the virus (using the VIC01 strain isolated by The Doherty Institute).
After studying SARS-CoV-2’s genome sequence, we’ve confirmed the virus is evolving into a number of distinct clusters in different parts of the world.
At this time, we do not think it will affect the development and evaluation of COVID-19 vaccines, therapies and diagnostics. But our scientists will continue to closely monitor the situation.
We’re also working with the Australian Government and Victorian manufacturers to build local capability and supply of materials. This will rapidly address the demand for personal protective equipment and other items for medical use.
- Forums
- ASX - By Stock
- MSB
- Cell Therapy News/Articles
Cell Therapy News/Articles, page-1631
-
-
- There are more pages in this discussion • 16,615 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
Add MSB (ASX) to my watchlist
 (20min delay) (20min delay)
|
|||||
|
Last
$1.13 |
Change
0.035(3.21%) |
Mkt cap ! $1.284B | |||
| Open | High | Low | Value | Volume |
| $1.09 | $1.13 | $1.07 | $3.910M | 3.535M |
Buyers (Bids)
| No. | Vol. | Price($) |
|---|---|---|
| 2 | 27197 | $1.11 |
Sellers (Offers)
| Price($) | Vol. | No. |
|---|---|---|
| $1.13 | 43578 | 3 |
View Market Depth
| No. | Vol. | Price($) |
|---|---|---|
| 4 | 31763 | 1.100 |
| 2 | 33264 | 1.090 |
| 2 | 18897 | 1.085 |
| 2 | 18926 | 1.080 |
| 1 | 18000 | 1.075 |
| Price($) | Vol. | No. |
|---|---|---|
| 1.130 | 23400 | 3 |
| 1.135 | 1090 | 1 |
| 1.140 | 65000 | 4 |
| 1.145 | 64500 | 3 |
| 1.150 | 92314 | 9 |
| Last trade - 16.10pm 27/09/2024 (20 minute delay) ? |
Featured News
| MSB (ASX) Chart |