Lol – I’m thinking that QRX has great potential to produce one of the funniest forums on HC. We certainly have some very polarised views.
I have never shorted a stock Oriol but I suspect that if we had learned we would be much wealthier.
Two ways as far as I know. Get on good terms with a broker in Australia (meaning that you have a lot of money) or set up an account in the US so that you can trade ADRs.
Whether it is good idea at the moment is hard to know. In the long term yes QRX has many problems and they may be insurmountable. But what of the short term.
Conventional wisdom has it that the market overshoots in both directions and many people short biotechs on a FDA approval (over-exuberance) and go long on failure (where the IP etc is over-sold).
Much might depend on how you see the gap down – was it a pro or novice gap. Its probably a mixture of both. The pro’s did get suckered by this just like the rest of us mugs. To a large extent – but not completely.
Some smart money sold into the “buy before decision” hype successfully – and if you look at an indicator like the chaiken money flow – before the decision money was flowing out. And some what we might call super smart money successfully shorted QRX.
In terms of the fundamentals I think there is little chance of QRX raising $70m to conduct a large P3 trial. And I don’t think they want to. Its much more likely that they will work out a plan with the FDA for first a small P2 trial which will refine safety measures as well as trying to provide some data around what size of safety margin represents clinical benefit. If this is successful then they will move to P3.
Now what is the theory for a safety advantage? In 2011 QRX presented at the world pain congress and their experts (two doctors) outlined two ideas. One was that because of efficacy synergies, less dose would be needed and therefore less side effects.
The second idea was that the combination acted on different opioid receptors and produced a synergy in its own right for safety. But it was believed that this safety synergy was less strong than the efficacy synergy. And if the safety advantage is harder to show then the sample needs to be larger with associated higher costs.
Given the very high risk in this scenario QRX needs to go back to P2a and start to show proof of concept. Find some sensitive measures that that they think have a sound theoretical basis and start showing that for equal dose there is a reliable signal being produced across a number of safety components. From this exploratory work you then move to a large confirmatory trial.
The difficult thing for the market here to get its head around is that while QRX will be saying that the further trials are to build on a body evidence the reality is that they are basically starting with a blank sheet of paper. And a P2a trial will make that all too painfully obvious.
Essentially a decade of work and $100m is worth very little – except for gaining experience the hard way that it pays to listen to the FDA. And some experience in getting trials up and running in the US fairly successfully and efficiently. Unfortunately they were the wrong trials.
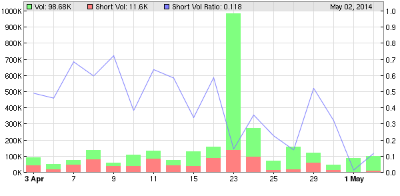
Anyway FWIW shown below short sales in the US. Not a lot of action. A bounce for QRX (dead cat or otherwise) wouldn’t surprise me. Assuming pro money has got stuck in this thing they will want to distribute their shares to the retail end. In theory this should be able to be spotted with price and volume rising but divergence on money flow and shorts in the US increasing.
Thanks to posters for their kind comments on other threads here.
Good luck to longs, shorts, dead cat bouncers, bottom drawers and the perplexed. Southoz[/URL]
Add to My Watchlist
What is My Watchlist?