I just received the following "spam" email...
I'm not interested of course, but thought some of you here on the OBJ thread might find it interesting.
Coppied the source code directly, so you could see the images...so hope it works.
Cheers!
---
Type II Diabetes is an epidemic in
the US- here are the hard numbers: 21 million Americans suffer from diagnosed
diabetes; 90% or 18 million have Type II Diabetes- Type II being less complex
version. Their bodies produce insufficient insulin, and they can be treated
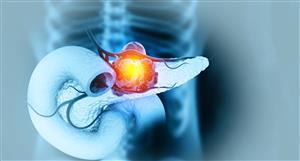
with insulin injections.Dermisonics is developing
a method for delivery of insulin transdermally- another words with a patch
rather than a needle. Transdermal delivery means through the skin- specifically
through a patch on the skin. Hence the image of a cross section of the
human skin for your benefit.Few drugs beyond birth control are
available in transdermal version for a very simple reason: The skin can
only absorb molecules with a molecular weight less than 500. Molecular
weights from 500 to 1,000 require some kind of enhancement to open the
pores. Currently, drugs with a molecular weight over 1,000 cannot be delivered
transdermally.There are only 14 FDA approved drugs
with a molecular weight of less than 500- Insulin has a molecular weight
of 6,000. Hence, the limitations of transdermal delivery for commonly taken
drugs despite the benefit of not having to use any needles or upset your
stomach.Enter Dermisonics (DMSI from
here forward). DMSI has a solution to the problem of transdermal
drug delivery. The company has discovered that the combination of a transdermal
patch and the application of very sophisticated ultra wave will open the
skin pores enough to allow the delivery of 82% of all drugs.
Using heat to open the skin pores
might seem like an obvious solution. However, with heat there are two problems:
1. The heated area can lead to skin irritation and 2. once exposed to heat,
the molecular structure of many drugs can change, making them ineffective.DMSI has discovered a way
to open the skin pores without heating the area. There are two components
to the skin patch. The white part you see in the picture is the transdermal
patch that contains the insulin. There is an adhesive around the outer
ring. This design prevents the adhesive from mixing with the medication.Once applied in the abdomen area
(the same place diabetics give insulin injections), the black component
snaps into place on the transdermal patch. This component provides the
ultrasonic signal which opens to skin pores and accepts the medication.
The ultrasonic component is attached
by wire to the unit you see pictured here. It will more likely be worn
on the belt like a cell phone rather than on the arm.This unit can be programmed to activate
the delivery of insulin either on command or on a regular schedule. It
has data memory, and can be hooked up to a computer so that the user can
send the usage data to his or her doctor over the internet.The unit also has an RFID port so
that future applications could include wireless commands.On Friday after the market closed,
DMSI
announced it would be starting Phase I clinical trials on this product
in the first qtr of '06. After lengthy deliberations with the FDA on the
design of a trial, the company is taking its first major step towards commercialization
of the technology.For the purposes of the regulatory
approval, their product is not considered a new drug and therefore the
process will not be as lengthy. It is considered a medical device. The
company will only have to prove the device delivers the drug effectively
without harming the user.DMSI will self finance the
study in Phase I clinical trials. A small sampling of patients with Type
II Diabetes will participate. In Phase II, a much larger sampling of at
least 250 patients will be used. Since this is a medical device, the company
can petition for its PMA (pre market approval) after the Phase II study
has been completed.
DMSI's timeline goals through
the first half of 2006 are as follows:
Commence the Phase I clinical trial
Complete and Compile Data from Trial
in the March/April time frame
Get peer review publication on data
from Associates- specifically hoping for Princeton University Medical
Unveil the product at the American Diabetes
Association annual convention in June
Seek out a major biotech or pharmaceutical
giant to partner up with the company for the Phase II clinical trial.
While there are no guarantees any or
all of the goals can be attained, the time line makes sense if they can
get the Phase I trial started in early 2006.On the fundamental side, DMSI
has 40 million shares I&O, leading to a $40 million market valuation
at $1. The company has $1.5 million in convertible debt, which converts
into a maximum of 600,000 shares based on the terms of the note and is
therefore, non toxic.It is certainly early stage and should
be looked at with a long term perspective. However, when one considers
the possibility for drug delivery of not only insulin, but the other 175
commonly used drugs that could be delivered transdermally with the DMSI
technology, the $40 million starting level offers an enormous upside.Patents also add value to the story.
DMSI
has filed for 15 patents on this process. One has been granted, and they
are closing in on a second. This will insure a "first to market" valuation
if and when the market prices in an approval.Technically there is nothing remarkable
about the chart. This is a weekly chart going back to mid summer. Like
so many of its micro brethren, the stock has been in a down trend early
August. Today's news release discloses that clincial trials are set to
start in the fall.I would expect the stock to start
getting traction as the company's unique transdermal/ultrasound delivery
system begins to get publicity in the medical community. This is very exciting
technology with unlimited upside. I don't know how long it will take, but
I can easily see this company being acquired by a major pharma or biotech
company down the road.Here is the complete text of today's
news for your review:
Press Release Source:
Dermisonics, Inc.Dermisonics to Begin
Next Key Phase of Pilot Trials of Revolutionary U-Strip Ultrasonic Drug-Delivery
TechnologyMonday December 12, 4:01
pm ETPioneering Non-Invasive
System Designed to Provide Insulin-Dependent Diabetics Painless Alternative
to InjectionsWEST CONSHOHOCKEN, Pa.--(BUSINESS
WIRE)--Dec. 12, 2005--Dermisonics, Inc. (OTCBB:DMSI - News; FWB:FQC), a
developer of painless, injection-free, ultrasonic transdermal drug-delivery
patches and technologies with broad pharmaceutical and consumer applications,
today announced that it will begin a new phase of human pilot trials to
test the use of its innovative U-Strip(TM) drug delivery system with a
study population of insulin-dependent Type-2 diabetics.The pilot trial, scheduled
to begin in January, 2006, is an essential milestone in the process to
develop, approve and commercialize the Company's unique drug delivery system,
according to Bruce Haglund, CEO of Dermisonics. These human pilot trials
will focus on the safety of the ultrasonic component of Dermisonics' U-Strip(TM)
technology."This trial is an exciting,
important component of the Company's Phase-1 Clinical Evaluations," said
Bruce Redding, Executive Vice President of Dermisonics. "Successful completion
of these trials will strengthen and accelerate Dermisonics' mission to
secure approval for the first-ever injection-free transdermal insulin delivery
technology, to capitalize on the burgeoning, $19 billion drug-delivery
market." The insulin-delivery marketplace alone is estimated at $5.4 billion.Dermisonics' completely
non-invasive, painless drug-delivery system uses ultrasonic technology
to expand the skin's pores, to enable heavy, large molecules to penetrate
the skin (that is, to be delivered transdermally) and enter the bloodstream.
Currently, most patients on regular drug regimens rely on often-painful
needle injections or catheter drug pumps.In the pilot trial, Dermisonics'
U-Strip(TM) transdermal drug delivery system will be tested on human volunteers
to evaluate the sensitivity of the skin to low-power ultrasound. Ultrasonic
technology is an integral part of the Company's unique drug-delivery solution,
designed to deliver large-molecule medications through the skin's naturally
occurring pores and hair follicles.In the newly announced
trial, more than two dozen Type-2 diabetic volunteers will wear blank U-Strip
transdermal patch systems, powered by low-powered ultrasound over a 5-hour
testing period. The study, known as the HPT4 pilot trial, is intended to
demonstrate that the continuous use of low-powered ultrasound will present
no adverse reactions or skin irritation to patients.When final regulatory
approval has been obtained, the U-Strip(TM) Insulin Patch technology has
the potential to improve the lives of both Type-1 and Type-2 diabetics.
Approximately 55 million of the 185 million diabetics worldwide are insulin-dependent,
and must endure painful needle injections to survive this debilitating
disease.About Dermisonics, Inc.
Dermisonics is an intellectual
property company and advanced technology incubator that is primarily focused
on the ongoing development, testing and eventual commercialization of a
transdermal patch that has been designed to facilitate the efficient and
needle-free delivery of drugs with large molecular structures into the
bloodstream. Its breakthrough system, called the U-Strip, is based on a
radical integration of microelectronics and ultrasonic science with a product-carrying
patch, and represents a quantum leap in non-invasive, transdermal delivery
technology. Tests have shown that this system facilitates the transdermal
delivery of insulin as well as potentially at least 175 other existing
drugs that at present cannot be effectively delivered through the pores
of the skin using conventionally available transdermal technology due to
their large molecular size. The Company has also developed other portable
ultrasonic systems for applications in the medical (Antiseptic Wand) and
skin care (U-Wand) fields. For more information visit http://www.Dermisonics.com.
For more investor-specific information about Dermisonics, please visit
http://www.trilogy-capital.com/dmsi_summary.aspx. To read or download an
Investor Fact Sheet about the Company, visit http://www.trilogy-capital.com/tcp/dermisonics/factsheet.html.
For real-time stock price quotes, visit http://www.trilogy-capital.com/tcp/dermisonics/quote.html.
Dermisonics is also traded on the Frankfurt, Germany, stock exchange under
the symbol FQC.Forward-Looking Statements
This release contains
forward-looking statements within the meaning of Section 27A of the Securities
Act of 1933 and Section 21E of the Securities Exchange Act of 1934 that
are based upon current expectations or beliefs, as well as a number of
assumptions about future events. Although the Company believes that the
expectations reflected in the forward-looking statements and the assumptions
upon which they are based are reasonable, it can give no assurance that
such expectations and assumptions will prove to have been correct. The
reader is cautioned not to put undue reliance on these forward-looking
statements, as these statements are subject to numerous factors and uncertainties,
including but not limited to adverse economic conditions, intense competition,
lack of meaningful research results, entry of new competitors and products,
adverse federal, state and local government regulation, inadequate capital,
unexpected costs and operating deficits, increases in general and administrative
costs, termination of contracts or agreements, technological obsolescence
of the Company's products, technical problems with the Company's research
and products, price increases for supplies and components, litigation and
administrative proceedings involving the Company, the possible acquisition
of new businesses or that result in operating losses or that do not perform
as anticipated, unanticipated losses, the possible fluctuation and volatility
of the Company's operating results, financial condition and stock price,
losses incurred in litigating and settling cases, dilution in the Company's
ownership of its business, adverse publicity and news coverage, inability
to carry out research, development and commercialization plans, loss or
retirement of key executives and research scientists, changes in interest
rates, inflationary factors, and other specific risks. In addition, other
factors that could cause actual results to differ materially are discussed
in the Company's most recent Form 10-QSB and Form 10-KSB filings with the
Securities and Exchange Commission.Contact:
Dermisonics, Inc.
Bruce Haglund, 888-401-DERM
(3376) (Toll Free)
610-543-0800
Fax: 610-543-0688
[email protected]
or
Trilogy Capital Partners
(Financial Communications)
Paul Karon, 800-592-6067
[email protected]
or
European Investor Relations
Contact
Michael Drepper, +49-621-430-6130
[email protected]Source: Dermisonics,
Inc.
- Forums
- ASX - By Stock
- WFL
- competition...???
competition...???
-
-
- There are more pages in this discussion • 3 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
Add WFL (ASX) to my watchlist
 (20min delay) (20min delay)
|
|||||
|
Last
0.3¢ |
Change
0.000(0.00%) |
Mkt cap ! $1.478M | |||
| Open | High | Low | Value | Volume |
| 0.0¢ | 0.0¢ | 0.0¢ | $0 | 0 |
Featured News
| WFL (ASX) Chart |
Day chart unavailable
The Watchlist
I88
INFINI RESOURCES LIMITED
Charles Armstrong, CEO & Managing Director
Charles Armstrong
CEO & Managing Director
SPONSORED BY The Market Online