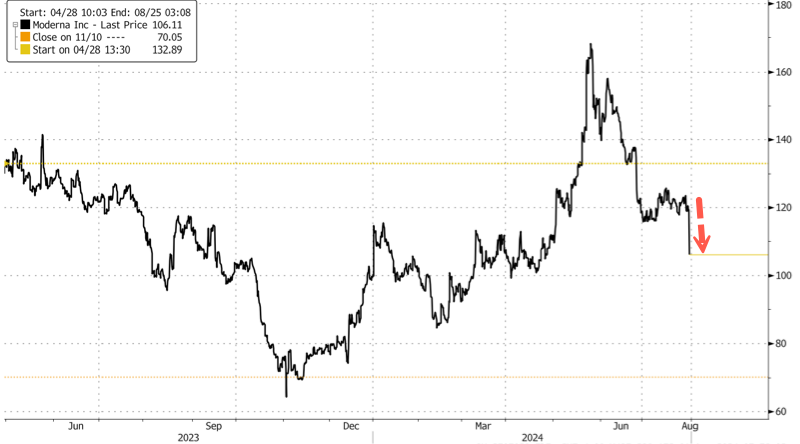
Moderna Shares Tumble 12% After Full-Year Sales Guidance Slashed
by Tyler DurdenThursday, Aug 01, 2024 - 01:25 PMShares of Moderna tumbled in premarket trading in New York after the biotech slashed its full-year sales guidance. The company cited lower sales in Europe, potential revenue deferrals for certain international sales into 2025, and an "increasingly competitive environment" for respiratory vaccines in the US.
Moderna posted second-quarter earnings that were narrower-than-expected losses and revenue that exceeded the average analyst estimate tracked by Bloomberg. It also posted a loss of $1.28 billion, or $3.33 per share, for the quarter, compared to a net loss of $1.38 billion, or $3.62 per share for the same period one year ago.
Snapshot of second quarter results (courtesy of Bloomberg):
Loss per share $3.33
Revenue $241 million, -30% y/y, estimate $131 million
Covid-19 vaccine revenue $184 million, estimate $106 million
Total operating expenses $1.60 billion, -27% y/y, estimate $1.63 billion
Cost of goods sold $115 million, -84% y/y, estimate $72.2 million
R&D expenses $1.22 billion, estimate $1.1 billion
SG&A expense $268 million, -19% y/y, estimate $307.7 million
Moderna noted that revenue slumps were primarily due to a transition of the seasonal Covid vaccine market. This is where Covid vaccine demand is lower in spring and generally rises in the fall and winter periods. However, CEO Stephane Bancel said that the demand for vaccines was a "good spring season" for the elderly population in the US.
The focus on earnings was Moderna's downshift in the full-year product sales guidance from $4 billion to $3 billion to $3.5 billion.
"The update in product sales is driven by three primary factors: very low EU sales in 2024, potential revenue deferrals for certain international sales into 2025, and an increasingly competitive environment for respiratory vaccines in the US," the company said.
CEO Bancel added, "During the second quarter, we marked the approval of our second mRNA product and signicantly lowered our operating costs. We remain focused on execution for the 2024-25 COVID season and the launch of our RSV vaccine in the US."
Shares of Moderna plunged as much as 12% in premarket trading.
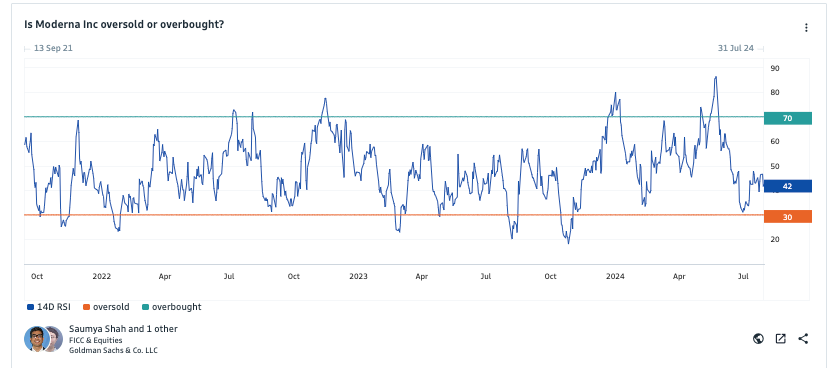
Goldman anylsts have been keeping an eye on Modern's selloff...
About a month ago, the Biomedical Advanced Research and Development Authority (BARDA) granted Moderna $176 million to develop bird flu vaccines. Now, Moderna is banking on a spike in human-to-human bird flu cases.
- Forums
- Breaking News
- COVID AND THE VACCINE - TRUTH, LIES, AND MISCONCEPTIONS REVEALED
COVID AND THE VACCINE - TRUTH, LIES, AND MISCONCEPTIONS REVEALED, page-99694
-
- There are more pages in this discussion • 3 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
MND
Albemarle lithium downsize burns $200M hole in Monadelphous's pocket as latter's contracts terminated
TG1
TechGen Metals kicks off airborne geophys survey at Sally Downs copper play – a first for the permit