End-of-Phase 2 Meeting
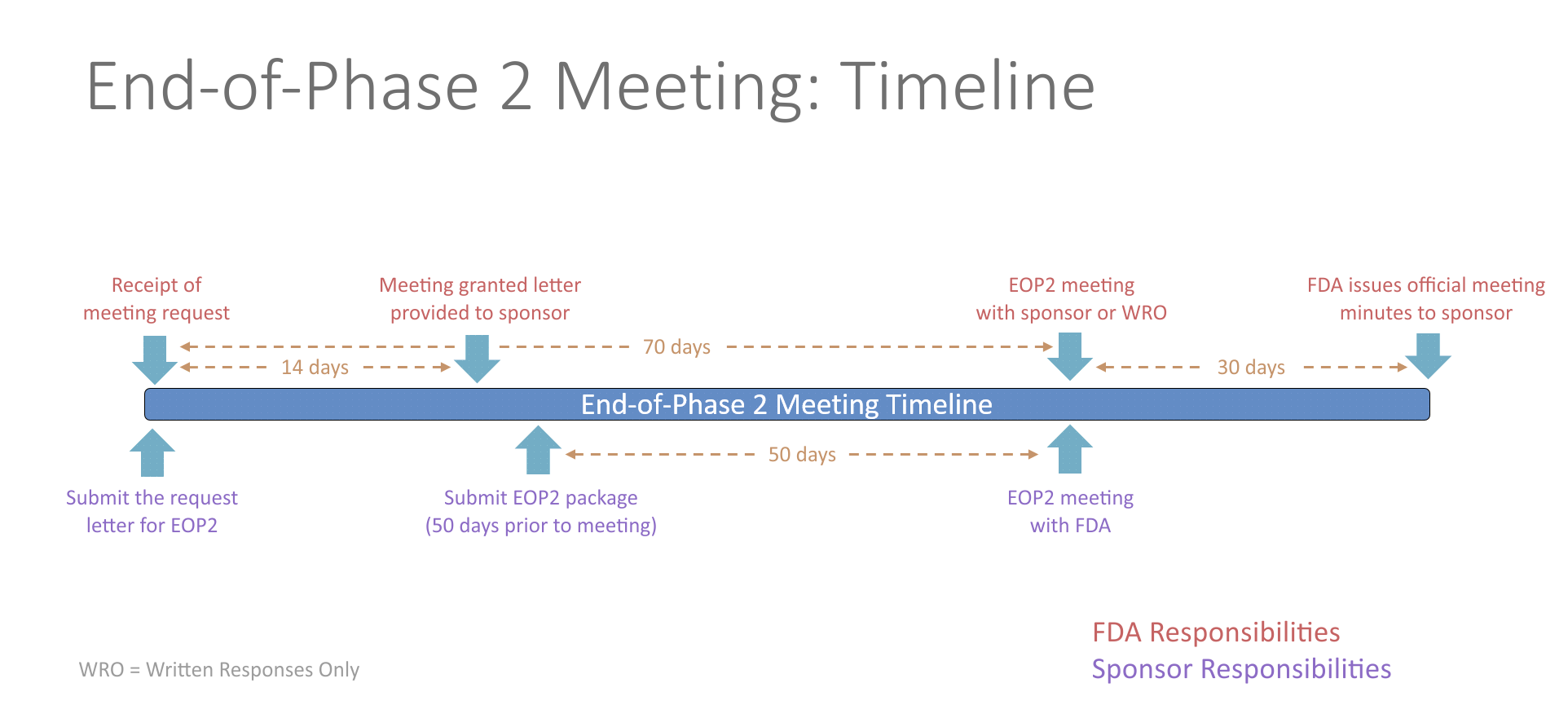
An end-of-Phase 2 meeting (EOP2) is a formal meeting between the sponsor of an IND, the regulatory contact, and the FDA. The purpose of an EOP2 meeting is to determine the pathway for proceeding to a Phase 3 study, to evaluate the Phase 3 plan and protocol for adequacy, to assess pediatric safety and effectiveness, and to identify any additional information that would be needed to support a marketing application. This provides the sponsor an opportunity to get FDA input prior to conducting an expensive Phase 3 study.
The FDA will schedule the EOP2 meeting within 14 days of receiving the written meeting request. At least 50 days prior to the EOP2 meeting, the sponsor should submit a meeting package containing the plan for Phase 3, summaries of Phase 1 and 2 investigations, specific protocols for Phase 3 studies, plans for pediatric studies, and tentative labeling for the drug, if available. The initial meeting request and the meeting package should contain a proposed agenda and a list of questions. Any agreements reached during the meeting will be recorded in the FDA meeting minutes and provided to the sponsor. Studies conducted in accordance with the agreement will be presumed to be sufficient in objective and design for the purpose of obtaining marketing approval of the drug.
- Forums
- ASX - By Stock
- NEU
- End of Phase 2 Meeting with FDA - September 2024
NEU
neuren pharmaceuticals limited
Add to My Watchlist
1.59%
 !
$19.82
!
$19.82
End of Phase 2 Meeting with FDA - September 2024
Featured News
Add to My Watchlist
What is My Watchlist?
A personalised tool to help users track selected stocks. Delivering real-time notifications on price updates, announcements, and performance stats on each to help make informed investment decisions.
 (20min delay) (20min delay)
|
|||||
|
Last
$19.82 |
Change
-0.320(1.59%) |
Mkt cap ! $2.503B | |||
| Open | High | Low | Value | Volume |
| $20.39 | $20.83 | $19.70 | $21.46M | 1.066M |
Buyers (Bids)
| No. | Vol. | Price($) |
|---|---|---|
| 2 | 7078 | $19.80 |
Sellers (Offers)
| Price($) | Vol. | No. |
|---|---|---|
| $19.94 | 3500 | 1 |
View Market Depth
| No. | Vol. | Price($) |
|---|---|---|
| 1 | 1000 | 19.800 |
| 1 | 310 | 19.750 |
| 1 | 1000 | 19.710 |
| 2 | 10581 | 19.700 |
| 1 | 1000 | 19.650 |
| Price($) | Vol. | No. |
|---|---|---|
| 19.950 | 18749 | 1 |
| 20.000 | 400 | 1 |
| 20.200 | 5000 | 1 |
| 20.390 | 1000 | 1 |
| 20.400 | 1150 | 1 |
| Last trade - 16.10pm 19/09/2025 (20 minute delay) ? |
Featured News
| NEU (ASX) Chart |