http://www.medscape.com/viewarticle/832976
EDEN PRAIRIE , MN — A counterpulsation device that can be implanted using minimally invasive surgical techniques and doesn't touch the blood it is pumping could find use in outpatients with advanced heart failure, suggests a very small feasibility study[1]. The pump is envisioned as a substitute, in some cases, for left-ventricular-assist devices (LVADs), over which it appears to have a number of advantages.
Twenty patients with the device, who—despite optimal treatment including biventricular pacing (if eligible)—were in NYHA class 3 or ambulatory class 4, overall showed significantly improved NYHA functional class and quality-of-life scores at six months and one year. Six-minute-walk distance improved significantly by one year.
Fully half the group met the main safety end point of device-related adverse events. There were no neurological events or MIs; however, three patients died, all within six months, but after the first 30 days and only one from a device-related cause. Patients with severe renal function or aortic disease had been excluded from the study, conducted at seven US centers.
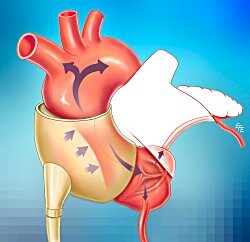
The C-Pulse (Sunshine Heart), which augments ventricular unloading and, in terms of invasiveness, seems to be somewhere in between intra-aortic balloon pumps long used in critical care and limb-encasing external counterpulsation devices, was designed for ambulatory use. Driven by a wearable, percutaneously connected power pack and pneumatic line, the pumping component consists of a cuff wrapped around the ascending aorta that inflates and deflates with the cardiac cycle. It may be the most advanced in clinical development of a number of counterpulsation devices, usually intra-aortic, proposed for outpatient use in heart failure.
"The non–blood-contacting feature of the C-Pulse system allows the device to be intermittently turned off as tolerated. This enables the patient to be 'untethered' from the device, allowing freedom for personal hygiene and convenience," according to the report's authors, led by Dr William T Abraham (Ohio State University, Columbus). The device, they write, "was intended to be used at least 20 hours per day."
Column 1 0 1 C-Pulse extra-aortic pump[Source: Ohio State University]
That the implanted device isn't immersed in blood also means that "no anticoagulants are required, reducing the risk of bleeding complications, and the extravascular nature of the implant mitigates the risk of intravascular thrombus formation, thromboembolism, and blood-borne infection."
An accompanying editorial[2] called the 40% drive line exit-site infection rate "disappointing" but the lack of strokes in the study "encouraging." Dr Carmelo A Milano (Duke University Medical Center, Durham, NC) writes, "While the results from Abraham et al could be discounted as unexciting, the chronic counterpulsation strategy in theory addresses several of the limitations currently being encountered with the more popular rotary-flow implantable left-ventricular-assist device systems."
Ten of the 20 patients in the C-Pulse IDE Feasibility Study , which had enrolled 12 men and eight women (13 with nonischemic and seven with ischemic heart disease), developed a device-related adverse event. That end point consisted of "death, major infection, aortic disruption, neurological dysfunction, myocardial infarction, or any other device-related adverse event," within six months after device implantation; its rate didn't change from month 6 to month 12.
"The composite adverse-event assessment was dominated by the incidence of manageable [drive-line] exit-site infections, which might be mitigated in the future by recently developed strategies for better drive-line fixation and management," according to the group. Eight of the 10 safety end points were exit-site infections, all of which led to hospitalization and one of which was judged responsible for the single device-related death. No patients were hospitalized for stroke, thrombosis, sepsis, or bleeding, "as is often observed with LVADs."
As secondary end points, mean NYHA class improved from 3.1 to 1.9 (p=0.0005) and quality-of-life scores improved significantly (<0.0005) by six months. Six-minute-walk distance improved significantly (p=0.0425) by 12 months but not by six months, and peak VO2 wasn't significantly changed at either point.
"On the basis of review of the feasibility-study data, a prospective, randomized, controlled trial designed to demonstrate and extend these observations was approved by the US Food and Drug Administration in November 2012 and is currently under way," according to Abraham et al.
The study was sponsored by Sunshine Heart, from which Abraham discloses receiving consulting fees. Milano discloses receiving consulting fees from Thoratec and HeartWare.
http://www.medscape.com/viewarticle/832976 EDEN PRAIRIE , MN — A...
Add to My Watchlist
What is My Watchlist?