Form F-1/A Advanced Human Imaging
November 17, 2021 6:05 AMAs filed with the Securities and Exchange Commission on November 16, 2021.
Registration Statement No. 333-259090
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 4 to
Form F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Advanced Human Imaging Limited.
(Exact Name of Registrant as Specified in its Charter)
Australia 7372 N/A 1 (State or Other Jurisdiction of
Incorporation or Organization)
(Primary Standard Industrial
Classification Code Number)
(I.R.S. Employer
Identification No.)
Vlado Bosanac
71-73 South Perth Esplanade
Unit 5
South Perth, WA 6151
Australia+61 8 9316 9100
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Joseph M. Lucosky, Esq.
101 Wood Avenue South, 5th Floor
Woodbridge, NJ 08830
Tel. No.: (732) 395-4400
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
Joseph M. Lucosky, Esq.
Lawrence Metelitsa, Esq.
Lucosky Brookman LLP
101 Wood Avenue South, 5th Floor
Woodbridge, NJ 08830
Tel. No.: (732) 395-4400
Fax No.: (732) 395-4401
Barry Grossman, Esq.
Sarah Williams, Esq.
Matthew Bernstein, Esq.
Ellenoff Grossman & Schole LLP
1345 Avenue of the Americas, 11thFloor
New York, NY 10105
Tel: (212) 370-1300
Fax: (212) 370-7889Approximate date of commencement of proposed sale to the public: As soon as practicable after effectiveness of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging growth company ☒ If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised accounting standards†provided to Section 7(a)(2)(B) of the Securities Act. ☐
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
CALCULATION OF REGISTRATION FEE
Title of Securities to be Registered(1) Proposed
Maximum
Aggregate
Offering
Price(2) (3)Amount of
Registration
Fee(4)(5)Units consisting of: (i) Ordinary Shares, no par value, represented by American Depositary Shares US$ 12,650,000 US$ 1,172.65 (ii) Warrants to purchase American Depositary Shares(6) - - Ordinary shares underlying the American Depositary Shares issuable upon exercise of the Warrants US$ 6,325,000 US$ 586.32 Underwriter’s Warrants to purchase American Depositary Shares(4)(7) - - Ordinary Shares underlying the American Depositary Shares issuable upon exercise of Underwriter’s Warrants(4) US$ 1,138,500 US$ 105.53 Total US$ 20,113,500 US$ 1,864.50
(1) All Ordinary Shares in the offering will be represented by American Depositary Shares, or ADSs, with each ADS representing 8 Ordinary Shares. ADSs issuable upon deposit of the Ordinary Shares registered hereby are being registered pursuant to a separate registration statement on Form F-6. (2) Pursuant to Rule 416, the securities being registered hereunder include such indeterminate number of additional securities as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. Includes Ordinary Shares that are issuable upon the exercise of the underwriters’ option to purchase additional ADSs. (3) The fee is calculated by multiplying the aggregate offering amount by 0.0000927, pursuant to Section 6(b) of theSecurities Act of1933. (4) Estimated solely for purposes of calculating the registration fee pursuant to Rule 457(o) under the Securities Act the Warrants are exercisable at a per share exercise price equal to 120% of the public offering price per Unit. The proposed maximum aggregate offering price of the representative’s warrants is US$1,138,500, which is equal to 120% of US$948,750 (5% of US$18,975,000, including ADSs and/or Warrants sold to cover over-allotments, if any). (5) $1,696 previously paid. (6) Each warrant is exercisable for one ADS at an exercise price of US$____ per ADS in this offering, (which shall not be less than 105% of the public offering price per ADS). (7) No separate fee is required pursuant to Rule 457(i) of the Securities Act. The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell the securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting any offer to buy these securities in any jurisdiction where such offer or sale is not permitted. SUBJECT TO COMPLETION DATEDNOVEMBER 16, 2021
Advanced Human Imaging Limited.
1,000,000 Units consisting of
2,000,000American Depositary Shares
representing 16,000,000 Ordinary Shares and
Warrants to Purchase 1,000,000 ADSs
Representing 8,000,000 Ordinary Shares
This is the initial public offering in the United States of units, or Units, each consisting of two American Depositary Shares, or ADSs, representing ordinary shares of Advanced Human Imaging Limited, and one warrant to purchase one ADS, or each, a Warrant. For illustration purposes, we have assumed that each ADS will represent 8 Ordinary Shares, no par value, deposited with The Bank of New York Mellon, as depositary. We anticipate that the initial public offering price of the Units will be between US$10.00 and US$12.00 per Unit and the number of Units offered hereby is based upon an assumed offering price of $11.00 per Unit, the midpoint of such estimated price range (after giving effect to the Australian dollar/U.S. dollar exchange rate of US$0.74:A$1.00 as of November 4, 2021, and an assumed ADS-to-Ordinary Share ratio of 8-to-1). The Units have no stand-alone rights and will not be certificated or issued as stand-alone securities. The ADSs and Warrants are immediately separable and will be issued separately in this offering. Each Warrant offered hereby is immediately exercisable on the date of issuance at an exercise price of $ per Warrant (which shall not be less than 105% of the public offering price per ADS) and will expire three years from the date of issuance. The Warrants will not be listed for trading.
Prior to this offering, there has been no public market for the ADSs or the Warrants. We have reserved the symbol “AHI” for purposes of listing the ADSs on The Nasdaq Capital Market (“Nasdaq”) and we have applied to list the ADSs on the Nasdaq Capital Market. No assurance can be given that its application will be approved. In the event that the ADSs are not approved for listing on Nasdaq, we will not proceed with this offering. There is no established trading market for any of the Warrants, and we do not expect a market to develop. We do not intend to apply for a listing for any of the Warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the Warrants will be limited.
Our outstanding Ordinary Shares are trading on the Australian Securities Exchange, or ASX, under the symbol “AHI”. On November 4, 2021 the closing price for our Ordinary Shares was A$0.89 per share, equivalent to a price of US$0.659 per share (after giving effect to the Australian dollar/U.S. dollar exchange rate of US$0.74:A$1.00 as of November 4, 2021).
The final offering price per Unit in U.S. dollars will be determined through negotiations between us and the representatives of the underwriters and will be based, in part, on prevailing market prices of our Ordinary Shares on the Australian Securities Exchange, after taking into account market conditions and other factors. For a discussion of the other factors considered in determining the final offering price per Unit, see “Underwriting.”
The assumed ADS-to-Ordinary Share ratio used throughout this prospectus has been included for illustration purposes only. The actual ADS-to-Ordinary Share ratio may differ materially from the assumed ratio used in the prospectus and will be determined by the Board of Directors.
We are both an “emerging growth company” and a “foreign private issuer”, as defined under the U.S. federal securities laws, and as such may elect to comply with certain reduced public company reporting requirements for this and future filings. See “Prospectus Summary—Implications of Being an Emerging Growth Company” and “Prospectus Summary—Implications of Being a Foreign Private Issuer.”
Investing in the ADSs involves a high degree of risk, including the risk of losing your entire investment. See “Risk Factors” beginning on page 20 to read about factors you should consider before buying ADSs.
Per Unit Total Public offering price US$ US$ Underwriting discount(1) US$ US$ Proceeds to us, before expenses(2) US$ US$
(1) See “Underwriting” in this prospectus for more information regarding our arrangements with the underwriter.
(2) The total estimated expenses related to this offering are set forth in the section entitled “Underwriting.”
Neither the Securities and Exchange Commission (“SEC”) nor any state securities commission nor any other regulatory body has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
We have granted the representative of the underwriters an option, exercisable within 45 days from the date of this prospectus, to purchase from us, up to an additional 300,000 ADSs at a per price ADS of $5.49 and/or up to an additional 150,000 Warrants to purchase 150,000 ADSs at a price per Warrant of $0.02 less, in each case, the underwriting discounts and commissions, to cover over-allotments, if any. If the representative of the underwriters exercises the option in full, the total underwriting discounts and commissions payable will be $132,000, and the total proceeds to us, before expenses, will be $1,518,000.
The underwriter expects to deliver the securities comprising the Units against payment in New York, New York on , 2021.
Sole Book-Running Manager
Maxim Group LLC
Prospectus dated , 2021
Advanced Human Imaging.
Beyond Measure.
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus or in any related free-writing prospectus. We and the underwriter have not authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses prepared by us or on our behalf or to which we have referred you. We take no responsibility for and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the Units offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. We are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted or where the person making the offer or sale is not qualified to do so or to any person to whom it is not permitted to make such offer or sale. We have not taken any action to permit a public offering of the Units outside the United States or to permit the possession or distribution of this prospectus outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to the offering of the Units and the distribution of the prospectus outside the United States. The information contained in this prospectus is current only as of the date on the front cover of the prospectus. Our business, financial condition, results of operations and prospects may have changed since that date.
We are incorporated under the laws of the Commonwealth of Australia and a majority of our outstanding securities are owned by non-U.S. residents. Under the rules of the SEC, we are currently eligible for treatment as a “foreign private issuer,” or FPI. As an FPI, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as domestic registrants whose securities are registered under theSecurities Exchange Act of 1934, as amended, or the Exchange Act.
i
ABOUT THIS PROSPECTUS
You should rely only on the information contained in this prospectus. We have not, and the underwriters have not, authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the underwriters are not, making an offer to sell securities in any jurisdiction where such an offer or sale is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front of this prospectus.
For investors outside of the United States of America (the “United States” or the “U.S.”): Neither we nor the underwriters have done anything to permit the conduct of this offering or the possession or distribution of this prospectus in any jurisdiction, other than the United States, where action for any such purpose would be required. Persons outside of the United States who come into possession of this prospectus must inform themselves about, and observe, any restrictions relating to the conduct of this offering and the possession and distribution of this prospectus that apply in the jurisdictions outside of the United States relevant to their circumstances.
Unless otherwise indicated or the context otherwise requires, all references in this prospectus to “the Company,” “AHI,” “Advanced Human Imaging,” “we,” “us,” and “our” refer to Advanced Human Imaging Limited. and its consolidated subsidiaries. Our direct corporate predecessor is MyFiziq Ltd., a limited company organized under the laws of Australia, and listed on the Australian Stock Exchange. On March 5, 2021, we changed our name to Advanced Human Imaging Limited.
In this prospectus, unless otherwise stated, all references to “U.S. dollars,” “USD,” or “US$” are to the currency of the United States of America, and all references to “Australian Dollars,” “AUD,” or “A$” are to the currency of Australia. Our presentation currency of the financial statements was AUD and will remain AUD. Throughout this prospectus, all references to “ADSs” mean American Depositary Shares, each of which represents of our Ordinary Shares, no par value.
This prospectus and the information incorporated herein by reference contain market data, industry statistics and other data that have been obtained from, or compiled from, information made available by third parties. We have not independently verified their data.
You should rely only on the information that we have provided or incorporated by reference in this prospectus. We have not authorized anyone to provide you with different information.
In this registration statement, any reference to any provision of any legislation shall include any amendment, modification, re-enactment or extension thereof. Words importing the singular shall include the plural and vice versa, and words importing the masculine gender shall include the feminine or neutral gender.
PRESENTATION OF FINANCIAL INFORMATION
The financial information contained in this prospectus derives from our audited consolidated financial statements in AUD as of June 30, 2021 and 2020. These financial statements and related notes included elsewhere in this prospectus are in the form of Australian Dollar (AUD or A$) and are collectively referred to as our audited consolidated financial statements herein and throughout this prospectus. Our audited consolidated financial statements are prepared in accordance with International Financial Reporting Standards, or “IFRS,” as issued by the International Accounting Standards Board, or “IASB”. Our fiscal year ends on June 30 of each year, so all references to a particular fiscal year are to the applicable year ended June 30. None of our financial statements were prepared in accordance with generally accepted accounting principles in the United States.
ii
The following summary is qualified in its entirety by, and should be read in conjunction with, the more detailed information and financial statements included elsewhere in this prospectus. In addition to this summary, we urge you to read the entire prospectus carefully, especially the risks of investing in the Units, discussed under “Risk Factors,” before deciding whether to buy the Units.
Overview
We have developed and patented a proprietary measurement/dimensioning technology that enables a User to check, track, and accurately assess their body dimensions privately using only a smartphone. We refer to this physical measurement and analytics tool as “BodyScan.” We have global customers/partners (“Partners”) who utilize our technology through a Software Development Kits (“SDKs”). Our global Partners have substantial audiences that they address, and from those underlying audiences, individual User (“User(s)”) will sign up for, or be given access to, the Partners’ software programs/apps that embed our technology components. Our global Partners currently include companies within the following sectors: (i) mobile health (“mHealth”), Telehealth, and Wellness; (ii) Life and Health Insurance; (iii) Fitness; and (iv) Consumer and Apparel.
Our patented technology allows our Partners to supply to individual Users, via our automated technology, the ability to take a series of images of themselves using a smartphone, which delivers accurate and repeatable measurements across an individual’s entire body. These measurements allow the individual to understand his/her dimensions and the physical changes that they are undergoing through diet, exercise and lifestyle modification. Further, the images that we capture also provide the individuals with an understanding of their potential health risks related to certain chronic diseases (including obesity and diabetes) and using the global standards measurements set by the World Health Organization (“WHO”), and the International Diabetes Federation (“IDF”). Once the image capture sequence is completed, it supplies those measurements to the Partner’s application, whose contract with the User then determines the manner in which it analyzes/reports the data and/or the potential health risk to the User. We are working towards globalizing our technology in order to assist individuals, communities and populations live healthier lives.
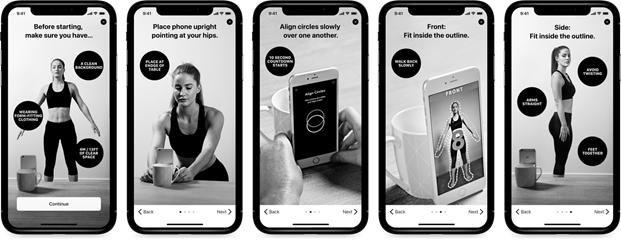
The above images illustrate the BodyScan capture process.
1
Recent technology advances have provided opportunities for complex mathematical problems to be solved directly on a User’s smartphone, rather than limiting that computation to the Cloud. Modern devices produced by companies such as Apple, Samsung and Google now have AI-focused chipsets utilizing platforms such as CoreML and Tensorflow to process data at lightning speeds. We see the opportunity to harness these ongoing technology improvements to lower latency, increase security and privacy, improve reliability and reduce operational costs of our core services. Our overarching technology strategy has been to take advantage of this hardware-accelerated performance, specifically by utilizing on-device general purpose Graphic Processing Units (“GPU”) found on today’s modern devices.
In Cloud-based systems, data transfer/retention is a potential impediment. Data must be sent to, and then processed in, the Cloud, thus adding additional latency and disclosure risk to the overall process. On-device computing eliminates the necessity of a roundtrip to the Cloud and permits near zero-latency. This process greatly improves User experience and allows for near real-time interaction with the service. Running directly on-device additionally negates the side-effects of Cloud-based interference. In areas where connectivity is sub-optimal, such as rural areas, having analytic models on-device means that processing results can be generated locally, quickly and securely.
As sensitive data does not need to be sent or maintained in the Cloud, there are fewer opportunities to exploit any potential vulnerabilities, thereby providing increased security and privacy for Users. This security is critically important in a world where data sovereignty, residency, and retention are a major concern for Users and under increased protective global legislation.
By focusing on leveraging the estimated 3.7 billion devices capable of running AI inference and analysis on-device, we are able to slash the costs associated with Cloud-based analytics and inference, bandwidth and retention/storage concerns. As our user base scales, implementing machine learning on-device will mitigate the expense of expertise and time needed to implement and maintain a Cloud-based solution.
We deliver a non-invasive, highly accurate and privacy-sensitive healthcare and biometric solutions that generate results to the User within seconds. We leverage machine-learning and computer vision to analyze images, detect pose and joint features, and create non-personally identified data for measurement estimation. We further take advantage of dedicated GPU libraries such as TensorFlow Lite (Android) and Metal (Apple) to run prediction models, which have been trained with a substantial and diverse human data set from around the globe and which are able to process multiple captured images in fractions of a second. The result is a solution that runs on-device and does not sacrifice speed, security or privacy. Images and private information never leave the phone, ensuring security and privacy standards are met across global regions. This process allows us to produce what we believe to be exceptional results and simplify the output of useful, reliable, digital measurements and remove the human error otherwise present in traditional methods, such as tape measure or visual estimations.
2
OurBodyScanapplication has been developed over 7 years through the development of our proprietary image capturing and analysis system. We have refined this process utilizing a proprietary data collection exercise conducted by the company, which involved over 7,000 individuals across Australia, Taipei, Thailand and Malaysia. This multinational data set of ethnicities was used to train and to enrich our machine-learning protocols and to improve the accuracy to an average 97.5% and repeatability to an average of 98% when using theBodyScansystem. We have further enhanced the use case by adopting a number of predetermined and published markers for some chronic diseases, set by the WHO, and the IDF. According to the WHO, these chronic, non-communicable diseases relate to 71% of deaths each year. We have developed and built the application’s patented capturing system, in line with the WHO and IDF measurement guidelines for the assessment and identification of biomarkers of these chronic diseases, such as Type 2 Diabetes. Whether it be an iOS or an Android application on the smartphone of any user, we provide our Partners and their Users with the following biometric data points:
● Anthropometric Measurements (BodyScan);
● Body Composition: Total Body Fat %, (BodyScan); ● Primary Markers of Chronic disease – Type 2 Diabetes, Obesity, Cardiovascular Disease (BodyScan and FaceScan combined);
● Primary Health Markers – Waist-to-Hip Ratio, Waist-to-Height, Waist Circumference, (BodyScan); and
● Dermatological conditions – 588 skin conditions across 133 categories, (DermaScan). It is our mission to deliver an easy-to-use, early warning and health assessment tool for our Partners to supply to individuals, governments and healthcare organizations, enabling Users to take control of and to understand the health risks which they pose to themselves, which they may be unaware of. Having the opportunity to combine measurement data with other biometric data sets widens the utility and importance of our technology through multiple business segments.
We believe that our technology is unique and has been independently validated for accuracy and repeatability by doctors and professors from leading universities and research organizations around the world, including Professor Timothy Ackland PhD, Professor of Applied Anatomy and Biomechanics, The University of Western Australia, Dr. Erwin Christianto MD MSc, Physician & Clinical Nutrition Specialist, Eka Hospital Pekanbaru Indonesia, and Dr. Alisa Nana PHD, Sports Science and Technology Mahidol University Thailand.
With the increased requirement of medical surveillance and remote healthcare services due to the COVID-19 pandemic, we have strategically partnered and invested into the expansion of our information capturing capabilities with the addition of vital signs data (FaceScan) using transdermal optical imaging (“TOI”) and also a further expansion into a dermatology AI platform which provides information to identify and assess 588 known skin conditions across 133 categories (DermaScan). DermaScanis a software application using artificial intelligence to perform patient-specific analyses of skin conditions.DermaScanis intended to be used as a second opinion tool for healthcare professionals to support the diagnosis of skin conditions but does not provide a definitive diagnosis. A definitive diagnosis should only be performed in a healthcare professional’s environment with the patient present. User error can potentially lead to limitations of the product asDermaScanrequires good quality images of the skin condition the user is analyzing as well as a series of questions to be answered specific to the user and the skin condition in question. It is important the user provides images of good quality and accurately provides information as prompted to do so. Accuracy of services will vary depending on the quality of image presented as well as the quality of this information provided.
3
Business Model
We operate a business-to-business (“B2B”) model and revenue is generated on both a subscription basis as well ason demand-use basis. The overall mercantile business model is one-to-one-to-many, whereby our sales channel customers are business Partners who have the relationship with the end User and whereby our technology is embedded into our Partner’s application that is made available to the Users on terms set by those two parties, thereby allowing User privacy (data on the User smartphone) and agreed data retention in the Partner’s ecosystem. We believe this B2B model enables lower overhead and allows AHI to leverage our Partners’ sales forces in an efficient and economical way. Our go-to-market strategy makes our business model highly scalable without the need for large corporate overhead, which cost-saving element will help to facilitate better operating margins as we increase the number of our Partners and as they scale the number of their Users. We have a pricing model which can be adjusted downward depending on scale (User volume) undertaken by the Partner.
Our technology has been both designed and developed to augment Partner applications via individual SDKs, enabling rapid integration opportunities for both native and hybrid solutions. Through the licensing of the use of our technology, Partners have the ability to effortlessly select the appropriate SDK components and then to customize these solutions to fit their own brands and needs. The Partner’s public cloud provider manages all of our Partners’ hardware and traditional software, including middleware, application software, and security. Accordingly, our offering allows flexibility and pricing scale reductions for our Partners as their User scale increases. AHI works with its Partners on the contractual remuneration and service basis involving a per-use, annual subscription or license fees. This choice is determined with the Partner at the time of engagement depending on the use case and User volume provided by the Partner.
With our technology and distribution channels now mostly developed, we are now entering into a growth phase. Serving as a catalyst for this growth, we now have 16 signed binding agreements with global Partners that we believe have a total of over 400 million potential Users within the following sectors: (i) mHealth, Telehealth, and Wellness; (ii) Life and Health Insurance; (iii) Fitness; and (iv) Consumer and Apparel.
We are selective in our choice of sales channel Partner, favoring companies who have a global outreach with large pre-existing User bases. When identifying a potential Partner, we take into account the current digital environment developed by the Partner and its applicability to the SDK offering we have developed. We also consider other factors such as available user base, market reach and time-to-market with the Partner’s digital team.
MultiScan Platform Capability
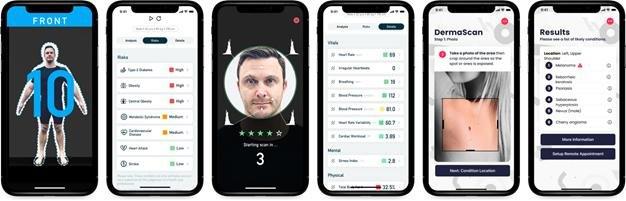
AHI offers a growing suite of human scanning solutions using a smartphone. This MultiScan Platform, called CompleteScan, currently includes BodyScan, FaceScan, and DermaScan.
Our MultiScan SDKs are embedded inside a Partner’s smartphone application(s) on both iOS and Android platforms to facilitate this multi-scan approach.
The MultiScan SDKs simplify many actions: User authorization, registering billing events, downloading remote assets, starting a new scan, returning results, and payment registration, all from a single interface/abstraction layer. These functions ensure that scans are easily integrated and correctly billed based on contractual agreements.
The above guide images various screens of the Multi-Scan suite.
4
Key MultiScan Technology Components
● BodyScan, supplied via our patented technology, provides body circumference, body composition (total body fat %), and specific health indicators relating to (including Type 2 Diabetes risk, as well as obesity and central obesity risk) which have a direct correlation to the chronic diseases we wish to assist our partners in managing. The Company is currently investigating the scope and requirements for the Food and Drug Administration (“FDA”) approvals should the need arise. However, as we are not a direct-to-consumer business, we are only providing the ability to capture the measurements. The way those measurements are used by a partner will determine if they require any approvals with the FDA. We have not engaged or commenced any formal process with the FDA at this time.
● FaceScan, supplied by a license with NuraLogix Corporation (“NuraLogix”), provides vital signs data, including blood pressure and heart rate, as well as health indicators, including cardiovascular disease risk, heart attack risk, and stroke risk. We have a technology use agreement and a reseller agreement with NuraLogix for the sale and distribution of our combined service offerings. The Company does not intend to seek regulatory approvals forFaceScanbut NuraLogix may seek FDA approval in the future.
● DermaScan, supplied by a license with Triage Technologies Inc. (“Triage”), provides skin disease detection for over 588 skin conditions across 133 major categories. Triage’s software application was approved as a class I medical device by Health Canada in June 2020. In April 2021, Triage received confirmation of medical device status in the European Union (EU) and is a CE marked medical device according to the Medical Devices Directive 93/42/EEC. Triage expects to seek FDA approval for its software application in the future. When combined into theCompleteScanplatform, the AI dermatology engine of Triage will be branded DermaScan and can be deployed to mobile devices to scan skin surface and then to assess the relevant skin conditions with simple images taken with that smartphone application.DermaScanis a software application using artificial intelligence to perform patient-specific analyses of skin conditions.DermaScanis intended to be used as a second opinion tool for healthcare professionals to support the diagnosis of skin conditions but does not provide a definitive diagnosis. A definitive diagnosis should only be performed in a healthcare professional’s environment with the patient present. User error can potentially lead to limitations of the product asDermaScanrequires good quality images of the skin condition the user is analyzing as well as a series of questions to be answered specific to the user and the skin condition in question. It is important the user provides images of good quality and accurately provides information as prompted to do so. Accuracy of services will vary depending on the quality of image presented as well as the quality of this information provided. Patented Technology
Our patented technology is the on-device image capture and data processing capability of BodyScanand each of our technology providers has its own set of patents.
Significant research and development (“R&D”) efforts by AHI have gone into optimizing, testing, and developing proprietary technology to work within smartphones’ confines. This R&D includes analyzing and processing phone sensor data, downloading assets remotely to reduce the initial resource size, utilizing hardware acceleration, and implementing machine learning libraries, such as TensorFlow and CoreML.
The result is a software that runs on the device without sacrificing speed, security, or privacy. Images and private information do not leave the device without explicit consent from the User to the primary app provider, ensuring global security and privacy concerns are met.
Data Points, Health Risks, and Health Indicators
Each scan returns a unique set of data which is categorized into three layers based on the type of data.
Layer 1 – Individual Data Points
These are the direct outputs from a scan, such as body circumference, diastolic and systolic blood pressure, and heart rate.
Layer 2 – Derived Data
Derived Data is a formula or equation applied to one or more data points. These are labeled “Health Indicators” and include waist-hip ratio, waist-height ratio, and a combined systolic/diastolic blood pressure result.
Layer 3 – Contextual Data
Contextual Data combines individual data points and derived data with a publicly available dataset or study used to predict health risk categorization. Examples include Type 2 Diabetes, Obesity, Hypertension, and Cardiovascular Disease.
5
As part of the partner integration progress, a data review is conducted to determine what scans and data are required. Documentation is provided to partners throughout this process to explain key concepts, including validation of measurements, disclaimers, research and studies, and understanding risk categorization.
It is important to note that the various scans offered within the MultiScan platform are not offered as a medical device nor as a pure diagnostic; moreover the image and data captures supply individualized data to the Partner in regard to their Users. Depending on the manner in which the Partner utilizes this data for health risk assessment will determine the Partner’s requirement to meet regulatory approvals in their operating jurisdictions.
How BodyScan Works
BodyScan’s image capture involves taking multiple front and side images of the individual. This process involves entering some basic personal details, such as height, weight, and gender, following a built-in guide provided upon setting up the phone, and then initiating a 10-second countdown for both the front and the side images. Images are processed on the phone and deleted upon the conclusion of the session.
This process is referred to as a “BodyScan” or a “Full Body Selfie”. The capture process utilizes “burst mode” or continuous shooting capabilities available in most cameras, and smartphones take multiple images using a timer.
Application of Our MultiScan Platform Technology to Multiple Business Segments
We have developed a MultiScan product strategy calledCompleteScancomprised of: BodyScan, FaceScan, and DermaScan, which unlocks a multitude of biometric markers and risk indicators that enables us to generate new layers of data, and cover a full spectrum of indicators for care including, but not limited to, cardiovascular, dermatological and chronic disease identification and prevention. We have designed this multiplatform with flexibility at its core, enabling each type of scan to be implemented either separately or in combination, depending on the partner’s and the user’s specific requirements. We believe that, due to our diverse and expansive offering, the company does not suffer from seasonality in regard to its use or appeal.
Examples of partner-specific requirements by business segment are: (i) mHealth, Telehealth and Wellness; (ii) Life/Health Insurance; (iii) Fitness; and (iv) Consumer Product and Apparel.
Growth Strategy: More Partners and More Users
With our technology and distribution channels now mostly developed; we are now entering into a growth phase. Serving as a catalyst for this growth, we have 16 signed binding agreements with global Partners that we believe have a total audience of over 400 million and we are targeting a total of 7 million users over the first 12 months of commercial launch within the following sectors: (i) mHealth, Telehealth, and Wellness; (ii) Life and Health Insurance; (iii) Fitness; and (iv) Consumer and Apparel. We believe that our existing Partners as well as prospective new Partner contracts will enable us to potentially grow to a run rate of 4.9 million paying Users in total within the next 3 years. This run rate is based on minimum agreed User targets between us and our Partners, for an initial 12-month period. We are targeting a penetration rate of 1.0% - 5.0% of the existing User base within each Partner company. Penetration assumptions have been modelled in accordance to known global statistics reported in the Liftoff mobile engagement index.
6
Our principal growth strategy is to leverage the ongoing and growing demand from existing and potential sales Partners for our technology, driven by global health concerns and by the various ways that the technology can assist with satisfying the need for fast, accurate and useful consumer/patient-centric data. The following factors will help us to commercialize our operations as we prepare for this next phase of growth:
● Contract Execution and Post-implementation Support. We have signed 16 binding agreements with various global Partners that we believe have a total audience of over 400 million. Our growth strategy includes carefully nurturing these agreements and tailoring the implementation of our technology with each partner to maximize the user experience. This process can be achieved by collaborating with our business partners to ensure that they are set up for success, including assisting them with the customer onboarding procedures and establishing feedback loops around user experience.
● Market Penetration. We believe that a key to our success lies in our ability to penetrate a variety of industries within multiple verticals. We intend to do so by identifying business partners who (a) are vetted for quality; (b) fit and audience reach; who already have a high volume of paying subscribers with a need for any or all of the components of the MultiScan platform being,BodyScan,FaceScan, orDermaScan, and (c) who can provide us with access to such clients/Users. Currently we operate in 4 major verticals & markets including: (i) mHealth, Telehealth, and Wellness; (ii) Life and Health Insurance Consumer; (iii) Fitness; and (iv) Consumer and Apparel. Over time we expect to further enhance our reach into each one of these verticals and markets as well as expand into other new ones.
● Ongoing Investment in Innovation. We intend to continue to invest in building and licensing new software capabilities and extending our platform to make our technology as accurate and “repeatable” as possible and to bring the power of accurate measurement to a broader range of applications. Achieving this goal will also serve as a barrier to entry for our competitors, both new and established. ● Intellectual Property Portfolio. Our issued patent portfolio covers ourBodyScantechnology and provides patent blocking and out-licensing opportunities, while the partner technologies we have licensed-in forFaceScanandDermaScanadds to ourCompleteScansuite of products. The company has furthered it protection by submitting additional patents for the combination of the proprietaryBodyScanwith vital signs measurements captured throughFaceScan. At this time, we have been unable to identify a direct, competitive product within the mobile phone ecosystem that: offers our range of measurement captures and data points across a similar multiplatform offering (BodyScan, FaceScan, DermaScan).Our Platform offers solutions across the four business segments in which we operate; and demonstrates competitive ease of use, accuracy and repeatability. Competitive Advantages
Our competitive advantage comes from our continuous innovation and transparency with regards to our data capture, measurement accuracy and repeatability metrics. We differentiate ourselves from our competitors through a multitude of factors including, but not limited to, the following:
● Ongoing Data Collection to Ensure Precise Digital Measurements.Regular, continuous, global data collections from multiple independent specialist organizations, including universities and hospitals, to ensure our measurement accuracy and repeatability remains the highest standard in digital measurements from a mobile phone. 7
● Continual Enhancement of our Technology Offering While Expanding and Refining our Patent Position. We currently maintain a robust intellectual property portfolio related to ourBodyScanTechnology, while technology we have licensed-in forFaceScanandDermaScanadds to our Complete suite of products. We regularly review and update our patents in key jurisdictions to prevent competitors from copying our image capture process or innovation. We believe further enhancing and protecting our patent portfolio is a key to the long-term success of our technology offering and business prospects. The company has lodged provisional patents covering the use case and the integrated use cases of the additional captures. ● Independent Validation of Accuracy and Repeatability of our Measurement Information.Publicly advertising our externally validated accuracy and repeatability metrics so that they are clear and accessible to our partners and competitors. Most of our competitors do not share clear information about their measurement accuracy and repeatability publicly. Our transparency makes it easier for potential Partners to understand our metrics during their solution selection process, significantly reducing the need for them to conduct testing and verification of our solution prior to purchase. ● Private, individualized Assessment on a Granular Personalized Basis.We measure the end User by taking images of segmented, non-personally identifiable set of silhouettes that are processed on-device. Our solution then recreates the User from the silhouettes in a virtual 3D environment by utilizing the Graphics Processing Unit (”GPU”) on the end User’s smartphone, enabling that User’s Personally Identifiable Information (“PII”) to remain on the device. As a result, the process is more cost effective, returns results faster and allows for better security of data and personal privacy.
● Broad Market Functionality. Due to high accuracy and repeatability of results from our product offering, we have been able to expand into multiple markets and uses. We intend to leverage and further grow our base of Partners through the four main large market segments in which we operate. ● Non-Opinionated Partner Level Integration. We offer a solution that, unlike many of our competitors, can be integrated into our Partners’ existing applications to match their own branding and User experience. This way, our technology becomes part of our Partners’ environment, allowing for trust and additional functionality for the User. ● Zero Upfront Integration Cost. Our potential Partners can implement our turnkey solutions using their internal development team, at an upfront cost of A$0, removing the need for an upfront capital budget that can often create a roadblock for many potential Partners. ● On-Device Processing Delivers Frictionless Scalability. We have adapted ourBodyScansoftware to run on a User’s device. This feature in turn only requires Amazon Web Services (“AWS”) for the tokenization of the event i.e. initiation of sequence to commence with all otherBodyScanfunctionality run on-device at no cost to us. It is important to note that allBodyScanmajor processing occurs on-device and as such AHI only uses AWS cloud infrastructure to support its services. These services include authorization of services (AWS Cognito), App configuration (AWS Cloudfront), and Billing events (AWS Serverless Aurora). For clarity AHI does not persist User data, nor does it preserve service results on its AWS cloud services, therefore the data stored within each of these services does not contain any User’s PII. ● Flexible and Capital Efficient B2B Business Model.We target potential B2B partners with large existing user bases, significantly reducing the costs of acquiring new Users in comparison to our competitors. We also offer our solution on a pricing tier, based on a per User per month basis whereby as User volume grows, Partners pay us less with each tier, enabling a higher return on their investment. 8
- Forums
- ASX - By Stock
- AHI
- Form F-1/A Advanced Human Imaging
Form F-1/A Advanced Human Imaging
-
- There are more pages in this discussion • 8 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
Add AHI (ASX) to my watchlist
 (20min delay) (20min delay)
|
|||||
|
Last
9.2¢ |
Change
0.000(0.00%) |
Mkt cap ! $22.60M | |||
| Open | High | Low | Value | Volume |
| 0.0¢ | 0.0¢ | 0.0¢ | $0 | 0 |
Featured News
| AHI (ASX) Chart |
Day chart unavailable