IDT acquired 23 FDA APPROVED Generics with Huge Market Potential ($460 Million) a few weeks ago ,and they have a own Cancer Drug a generic version of Temozolomide under review by FDA which is partnered with Mayne Pharma .
There is strong interest by potential commercialisation partners for the newly acquired Generics . IDT will bring these Drugs with a Partner or more back to the US-Market as soon as possible .
This Undiscovered stock is BRUTAL undervalued with a ridiculous Market Cap of $29 Million . This is minimum a Potential Disallowed Gem . GL
IDT Australia (IDT)
Market Cap: $29 Million
Cash: $7 Million
Price: $0.15
Burn-Rate: ~$1.4 Million per Quarter
Annual Rev: $13.5 Millionen
Product Presentation
http://idt.live.irmau.com/IRM/Compa.../InvestorInformationregardingANDAAcquisitions
As announced to the ASX on 3 November 2014, the new product suite comprises 23 tablet and capsule products for conditions including Parkinson’s Disease,depression, infection,hypertension and pain. The total current annual global market for these products in these presentations is approximately $460 million (IMS).
In parallel with the integration of these drugs into IDT’s manufacturing facility, the Company is also in discussions with over half a dozen potential commercialisation partners interested in US distribution of the products. Interest has been strong and indicative term sheets have already been received from several of these parties. It is the Company’s expectation that IDT will select one or more commercialisation partners and execute US distribution deal(s) for the initial cohort of products in early to mid 2015.
IDT Completes Transformational AcquisitionOf 23 US Generic Drugs..
http://idt.live.irmau.com/IRM/Compa...10000000/Acquisition23USGenericDrugscompleted
US distribution for its generic Temozolomide product ..
http://idt.live.irmau.com/IRM/Company/ShowPage.aspx/PDFs/1193-10000000/USproductdistribution
Temozolomide is indicated for the treatment of melanoma and glioblastoma multiforme and had US sales of approximately USD 340 million in the 12 months ending 31 May 2014 .
The Abbreviated New Drug Application (ANDA) submission for Temozolomide is the first of IDT’s generic filings (ASX announcement 18 November 2013). It has now been accepted for
review by the United States Food and Drug Administration (FDA) and is currently progressing through the FDA’s review process.
- Forums
- ASX - By Stock
- IDT-DD ..A Potential Disallowed Gem here !!!
IDT acquired 23 FDA APPROVED Generics with Huge Market Potential...
-
-
- There are more pages in this discussion • 4 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
Add IDT (ASX) to my watchlist
 (20min delay) (20min delay)
|
|||||
|
Last
13.0¢ |
Change
0.015(13.0%) |
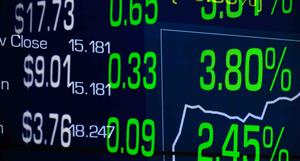
Mkt cap ! $55.84M | |||
| Open | High | Low | Value | Volume |
| 12.0¢ | 14.5¢ | 11.8¢ | $126.0K | 964.7K |
Buyers (Bids)
| No. | Vol. | Price($) |
|---|---|---|
| 1 | 24999 | 13.0¢ |
Sellers (Offers)
| Price($) | Vol. | No. |
|---|---|---|
| 13.5¢ | 8687 | 1 |
View Market Depth
| No. | Vol. | Price($) |
|---|---|---|
| 1 | 24999 | 0.130 |
| 2 | 12739 | 0.125 |
| 2 | 50999 | 0.115 |
| 5 | 158317 | 0.110 |
| 3 | 293894 | 0.105 |
| Price($) | Vol. | No. |
|---|---|---|
| 0.135 | 8687 | 1 |
| 0.140 | 250000 | 4 |
| 0.145 | 217629 | 5 |
| 0.150 | 305000 | 2 |
| 0.155 | 182635 | 2 |
| Last trade - 16.10pm 12/07/2024 (20 minute delay) ? |
Featured News
RNU
Renascor wins a funding boost given it wants to produce a critical mineral – but $5M award pales in comparison to some
FWD
Queensland's housing crisis an opportunity for ASX builder Fleetwood – and taxpayer cash a safe harbour from the storm
| IDT (ASX) Chart |