Advances in electroporation technology
We have spent over 20 years optimizing and innovating electroporation to bring you best-in-class electroporation technology, including:
- Electroporation buffer for all cell types
- Over 100 protocols for a variety of cell types
- Improved cuvette design with our Processing Assemblies
- Groundbreaking Flow Electroporation protocol to enable cGMP-compliant, large-scale genetic engineering
- Connectivity for closed system cGMP manufacturing
Understanding Flow Electroporation
Electroporation is a non-viral transfection technique that uses electricity to relax cell membranes, allowing payload to enter. Our Flow Electroporation technology is uniquely designed to allow cells to flow through the processing chamber where discrete volumes are electroporated, then collected on a continual basis. This pioneering innovation makes genetic engineering at large scale a reality.
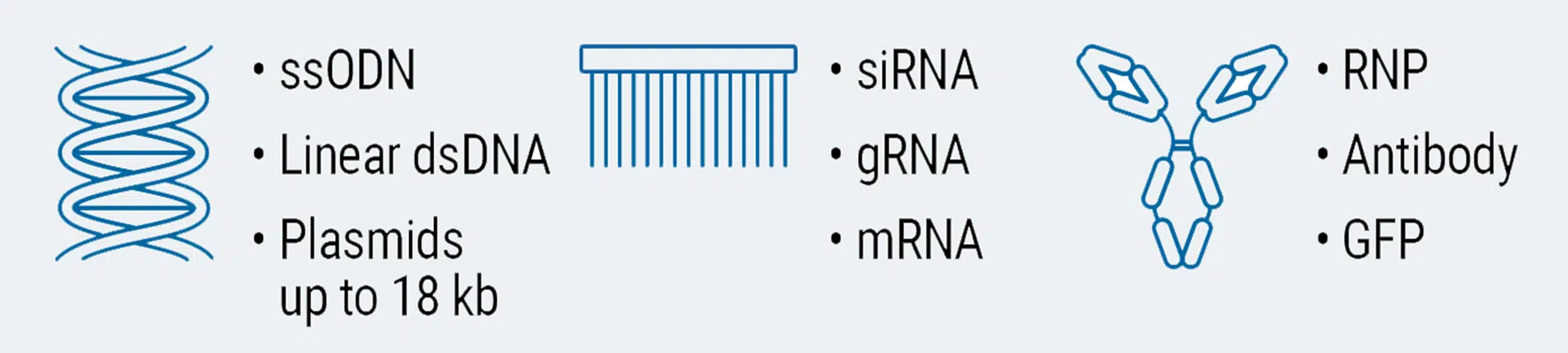
Load any molecule at any scale in any cell
Cells and Scalability
Whether you are at the beginning of the research journey transfecting thousands of cells or in the middle of scale-up looking to transfect billions of cells, MaxCyte is the best partner to help steer your project to success with our scalable electroporation technology.
With over 100 proprietary, optimized electroporation protocols for a broad range of cell types we’ve got you covered wherever your journey of discovery takes you!
Streamlined therapeutic development
MaxCyte has evolved the electroporation process from a single cuvette experiment to a Flow Electroporation® protocol ideal for genetic engineering at commercial scale. Higher transfection efficiency and increased cell viability compared to other approaches make Flow Electroporation perfectly suited for large-scale therapeutic manufacturing. And because the complete workflow, from development to manufacturing, can be performed on a single platform, there is no need for repeat optimization and validation when progressing from concept to clinic. This results in accelerated timelines with the added benefit of reduced manufacturing cost. A more rapid, reliable and safe therapeutic development pipeline can expedite your therapies to patients, saving lives.
Add to My Watchlist
What is My Watchlist?