The following was the most recent SA article written by Alpha Exposure which @Southoz refers to.
Mesoblast: Caught Red-Handed
Sep. 7, 2016 11:50 AM ET
|
8 comments
|
About: Mesoblast (MESO)
Alpha Exposure
Long/short equity
(1,782 followers)
Summary
We believe we have clear, undeniable proof that Mesoblast changed the reported endpoints in its Phase 2 Rheumatoid Arthritis trial.
MESO’s auditor just issued a going concern warning.
Mesoblast admitted it had a material weakness in financial reporting. How much should investors trust Mesoblast?
Proof Mesoblast Changed Endpoints
We believe we have clear, undeniable proof that Mesoblast (NASDAQ: MESO) changed the reported endpoints in its Phase 2 Rheumatoid Arthritis trial to make the results appear favorable.
Earlier this month, we wrote about how we believed the Phase 2 Rheumatoid Arthritis trial for Mesoblast had failed, and we documented how we believed MESO employed statistical sleight of hand, data-mining techniques, and post-hoc analysis to make it appear that the trial had succeeded (here). We pointed out that many of the reported endpoints that Mesoblast touted did not match the stated endpoints in the ClinicalTrials.gov page, and it appeared to us that Mesoblast had changed its statistical methodology. We believe this showed that Mesoblast had manipulated the trial data to make the trial appear successful. We obviously hit a nerve with the company because Mesoblast came out and protested our findings in both a hastily written press release ( here) and the FY June 2016 conference call (here).
In these recent public statements, the company swears that its statistical and analytical methods were kosher and did not include any adjustments for multiplicity, post-hoc analyses, or data-mined endpoints. However, we believe we caught MESO red-handed in altering a key reported endpoint in the Phase 2 RA trial, which is in direct conflict with its public statements. Investors can clearly see that Mesoblast changed the reported DAS28/CRP endpoint from the initial results announcement back in February to the final results release in August.
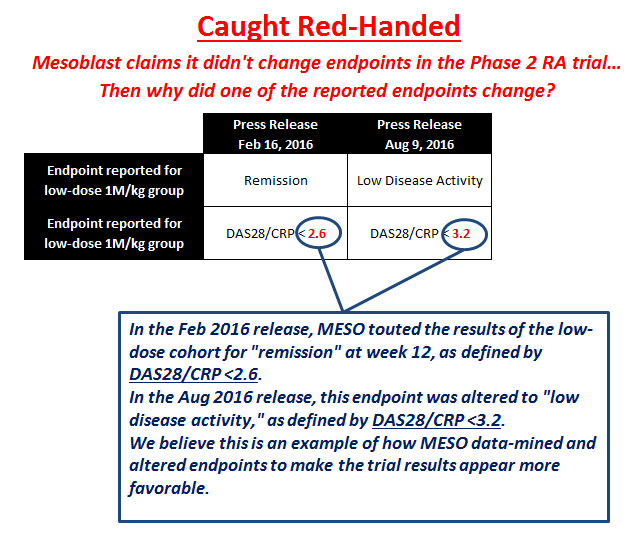
In the February 16, 2016, initial results press release (here), Mesoblast touts an endpoint called, "Remission at week 12, as defined by Disease Activity Score (DAS28/CRP) <2.6." In fact, Mesoblast highlighted this <2.6 endpoint as a "key top-line result" for the low-dose cohort.
If this is actually a key endpoint and part of the trial's pre-specified Statistical Analysis Plan (NYSE:SAP), then we should expect that same endpoint to be updated and announced as part of the final results release. We believe a sign of quality in a trial's results is consistency in the presented data. However, this "key top-line result" is nowhere to be found in the August 9, 2016, final results (here). Instead, Mesoblast only mentions an endpoint for "low disease activity state, defined as DAS28-CRP <3.2."
Not only has the DAS28/CRP endpoint been altered, but it's also a weaker finding than the <2.6 cut-off because it no longer represents disease remission. We believe this is undeniable proof that Mesoblast changed the reported endpoints in order to make the trial results appear more favorable.
Where did this DAS28/CRP <2.6 endpoint go, and why wasn't it reported again in the final results if it was a part of the pre-specified SAP? We believe the reason is clear: Mesoblast conducted post-hoc analysis and data-mined to find the most favorable results it could dredge up. When the DAS28/CRP <2.6 endpoint didn't turn out to be positive at the final reading, we believe Mesoblast manipulated the endpoint criteria to present something more favorable. We believe the Phase 2 RA trial failed and that Mesoblast is trying to fool investors into believing the trial was a success.
Going Concern Warning
Why do we believe Mesoblast is trying to fool investors into believing the Phase 2 RA trial was a success? We believe it is because Mesoblast needs to distract investors from its perilous financial position.
Mesoblast's FY June 2016 Preliminary Final Report from August 25, 2016 (here, see page 188) reveals that auditor PricewaterhouseCoopers issued a going concern warning.
We believe that this is a significant negative development for Mesoblast; and we feel that investors should be alarmed, as the 2015 Independent Auditor's Report (here, page 107) did not include a going concern warning. And, importantly, Mesoblast cannot claim that this was the result of a change in service providers as Mesoblast used the same auditor, "PricewaterhouseCoopers," and the same partner, Jon Roberts, signed off in 2016 as in 2015.
Material Weakness in Internal Controls Identified
In addition to a going concern warning issued by the auditor, investors should be aware that Mesoblast "determined there is a material weakness in the internal control over financial reporting." The disclosure can be found in the FY June 2016 Preliminary Final Report issued August 25, 2016 (here, page 128).
We believe that a going concern warning from the auditor along with disclosures of material weakness in financial reporting are serious problems that investors should be gravely concerned about.
Should Investors Trust Mesoblast?
This makes us question what, if anything, we can rely on from Mesoblast.
We know that ex-partner Teva Pharmaceuticals (NASDAQ: TEVA) abandoned the Phase 3 Chronic Heart Failure trial (here).
We believe the Phase 2 RA trial failed and that MESO performed post-hoc analysis to data-mine for positive endpoints (here).
We believe we have caught Mesoblast trying to mislead investors when the company said it had not altered the endpoints in the Phase 2 RA trial and data-mined for successful results (here).
We know MESO's auditor has sufficient doubts about Mesoblast's financial viability and accordingly, has issued a going concern warning.
Finally, we know that MESO has identified a material weakness in its internal controls over financial reporting.
New Price Target: $0
Given the recent developments and the warning issued by the auditor and the lack of financial reporting controls that Mesoblast itself identified, our new target price is $0.
Disclosure: I/we have no positions in any stocks mentioned, but may initiate a short position in MESO over the next 72 hours.
I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it. I have no business relationship with any company whose stock is mentioned in this article.
- Forums
- ASX - By Stock
- Mesoblast Phase 2 Trial Results Show Early and Durable Effects of Single Mesenchymal Precursor Cell
The following was the most recent SA article written by Alpha...
-
-
- There are more pages in this discussion • 92 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
Add MSB (ASX) to my watchlist
 (20min delay) (20min delay)
|
|||||
|
Last
$1.03 |
Change
0.080(8.42%) |
Mkt cap ! $1.176B | |||
| Open | High | Low | Value | Volume |
| 95.5¢ | $1.03 | 95.0¢ | $43.38M | 42.28M |
Buyers (Bids)
| No. | Vol. | Price($) |
|---|---|---|
| 4 | 72752 | $1.01 |
Sellers (Offers)
| Price($) | Vol. | No. |
|---|---|---|
| $1.03 | 205100 | 2 |
View Market Depth
| No. | Vol. | Price($) |
|---|---|---|
| 1 | 20000 | 1.005 |
| 5 | 57985 | 1.000 |
| 1 | 50000 | 0.995 |
| 2 | 20525 | 0.990 |
| 1 | 10000 | 0.985 |
| Price($) | Vol. | No. |
|---|---|---|
| 1.035 | 190122 | 7 |
| 1.045 | 39714 | 5 |
| 1.050 | 21696 | 6 |
| 1.060 | 9750 | 2 |
| 1.065 | 5780 | 1 |
| Last trade - 16.10pm 30/08/2024 (20 minute delay) ? |
Featured News
| MSB (ASX) Chart |
The Watchlist
1CG
ONE CLICK GROUP LIMITED
Mark Waller, MD
Mark Waller
MD
Previous Video
Next Video
SPONSORED BY The Market Online