I wanted to start this thread to discuss and collate information about MPC-06-ID since I believe these will be the next results to drop, even potentially by the end of the month. All contributions are welcomed, as long as it's relevant to MPC-06-ID phase 3 clinical trial.
All forms of discussion are encouraged, both in support and against the primary outcome being achieved. I believe the primary outcome will be achieved - change my mind.
So to get things started:
https://www.mesoblast.com/product-candidates/spine-orthopedic-disorders/chronic-discogenic-low-back-painClinical Trials
Ongoing Phase Trial 3
A Phase 3 clinical program has completed enrollment of 404 patients.
This Phase 3 clinical trial will use a primary endpoint that comprises both pain relief and improved function, consisting of a 50% reduction in lower back pain as measured by Visual Analog Score and a 15-point improvement in Oswestry Disability Index, with no additional interventions.
Find out more about this Phase 3 trial.
Phase 2 Trial
MPC-06-ID was evaluated in a randomized, placebo and active controlled Phase 2 trial conducted in 100 patients with more than six months of CLBP due to disc degeneration.
In March 2017, 36-month results were released and showed that a single intra-discal injection of 6 million MPCs resulted in meaningful improvements in both pain and function that were durable for at least three years. Previously, 24-month results were presented at the 24th Annual Scientific Meeting of the Spine Intervention Society in July 2016, and received the 2016 Best Basic Science Abstract award at the New Orleans meeting.
https://clinicaltrials.gov/ct2/show/NCT02412735?term=mesoblast&recrs=d&draw=2&rank=1
Primary Outcome Measures :
- Treatment Success (composite responder analysis of low back pain Visual Analogue Scale (VAS) score, Oswestry Disability Index (ODI) score and no post-treatment interventions) [ Time Frame: 24 Months ]
• To determine Overall Treatment Success of rexlemestrocel-L alone or rexlemestrocel-L+HA through 24 months based on a composite responder analysis
https://www.mesoblast.com/clinical-trial-results/mpc-06-id-phase-2Trial Results: MPC-06-ID Phase 2 Chronic Low Back Pain Due to Disc Degeneration Clinical Trial
With respect to the primary endpoint, allogeneic MPC treatment, including MPC-06-ID, was well tolerated with the most frequently reported adverse event, back pain, occurring across all patient groups.
With respect to primary efficacy endpoints, the FDA has provided guidelines on how to evaluate patient response, utilizing a composite endpoint based on achieving minimally important clinical differences, or MICD, in both pain and function from baseline. Such a composite endpoint for restorative or replacement disc therapies is different than that typically used by pharmacologic agents developed solely for palliative improvement in symptoms, such as analgesics, where short term improvement in mean pain scores between groups is sufficient to support a label for short term pain reduction. The FDA and key opinion leaders, or KOLs, have deemed that for restorative or replacement disc therapies the MICD for pain reduction should be at least a 30% improvement from baseline and for functional improvement at least a 30% improvement or 10 point improvement from baseline using a 100-point functional scale. We believe that achieving success in long-term improvement in both pain and function using even higher threshold levels than the MICD with durable outcomes for up to two years from a single dose should support a broad label for disc restoration and attractive pricing and reimbursement from payors.
We have utilized this composite-based endpoint and associated guidelines, among other measures, in the evaluation of our Phase 2 results.
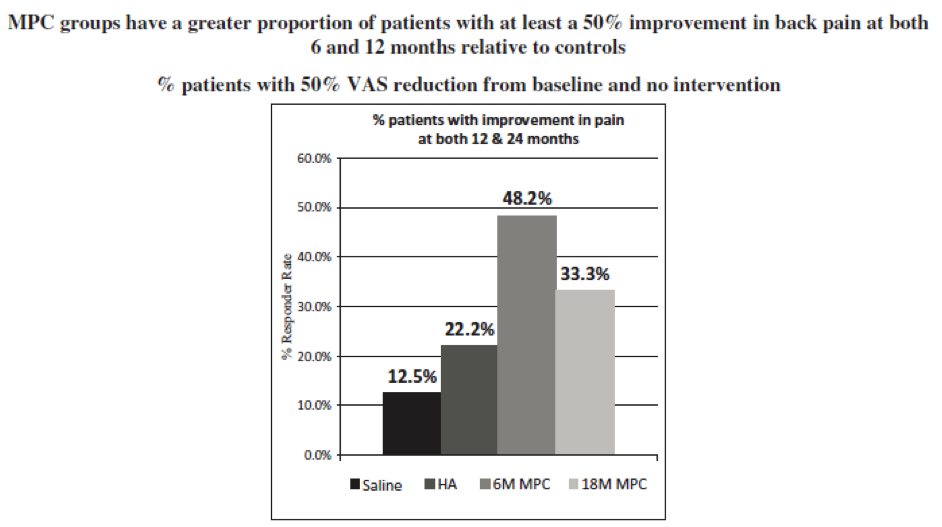
- Improvement in chronic low back pain. At 12 months, a responder analysis showed that there was clear separation between both treatment groups and both control groups at every decile increase in response beyond the MICD of 30% reduction in pain from baseline. In line with guidance from KOLs and from payers, a responder analysis was performed targeting at least 50% reduction in pain from baseline. At both 6 and 12 months, a reduction in pain from baseline of 50% or more, without any additional intervention, was seen in 59.3% of the MPC-06-ID group, 44.8% of the 18 million MPC group, 18.8% of the saline group, and 15.8% of the HA group, as measured by visual analog scale, or VAS (p = 0.006 across all four groups, p=0.023 for 6 million MPC against saline and p=0.006 against HA). Statistical significance denotes the mathematical likelihood that the results observed are real and not due to chance.
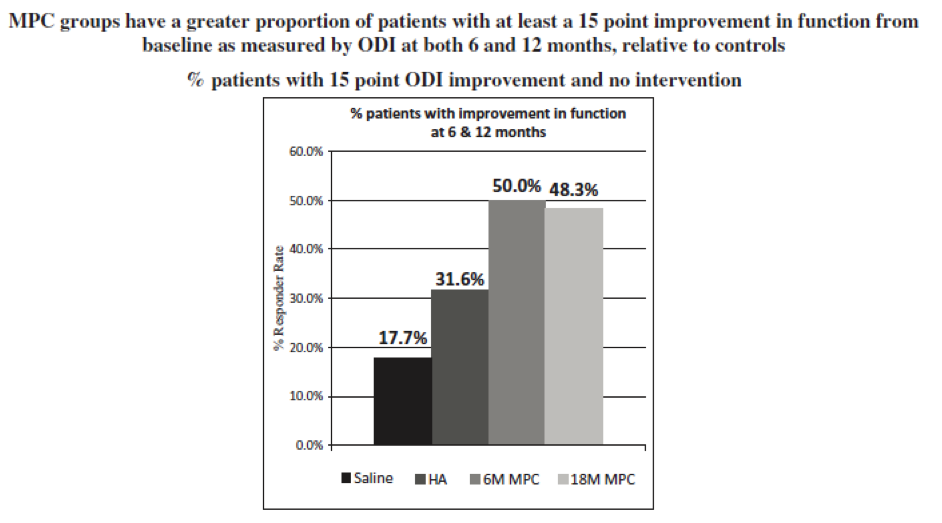
- Improvement in function: At 12 months, a responder analysis showed that there was clear separation between both treatment groups and both control groups at every decile increase in response at or beyond the MICD of 30% improvement in function from baseline. In line with historical FDA preference for spine fusion and artificial disc replacement marketing application approvals, a responder analysis was performed targeting at least a 15 point improvement in function through 24 months from baseline. At both 6 and 12 months, an improvement in function from baseline of 15 points or more, as measured by Oswestry Disability Index, or ODI, without any additional intervention, was seen in 50.0% of the MPC-06-ID group. 48.3% of the 18 million MPC group, 31.6% of the HA group, and 17.7% of the saline group (p=0.05 MPC-06-ID versus saline, p=0.06 18 million MPC versus saline).
- Reduced need for additional surgical and non-surgical interventions: MPC-treated patients had a significantly reduced need for additional interventions at the treated disc level, including surgical intervention (spinal fusion, discectomy or artificial disc replacement) or injection (epidural steroid injection, rhizotomy or transforaminal injections), than saline controls. By 12 months, 25% of patients in the saline control group had undergone an additional intervention, compared with 15% of patients in the HA control group, 6.9% of patients in the MPC-06-ID group and only 3.3% of patients who received 6 or 18 million MPCs. By Kaplan-Meier analysis of time to a first additional treatment intervention, treatment with either MPC-06-ID or 18 million MPC significantly reduced the need for additional interventions compared with saline treatment (p=0.024 and p=0.010, respectively).
- Radiographic measurements: In patients with early disc degeneration (Pfirrmann MRI degenerative grades below 5), increased translational movement of the disc is a potential indicator of instability associated with early disc degeneration and annular fissures seen on MRI and pathologic examination. This is an FDA validated measurement that has previously been used in Phase 3 trials of surgical devices for discogenic back pain. At 12 months, MPC-treated patients demonstrated a reduction in radiographically-determined translational movement of the disc, suggesting a treatment effect on disc degeneration, anatomy, and improved disc stability. The 18 million MPC group had a mean translational movement of only 1.3%, the MPC-06-ID group 2.0%, the HA group 2.5%, and the saline group 3.5% (p=0.021 between groups). In this study, 85% of patients had early disc degeneration as evidenced by Pfirrmann grade <5 on MRI. At 12 months, no significant differences were seen between groups in overall Pfirrmann grade by MRI.
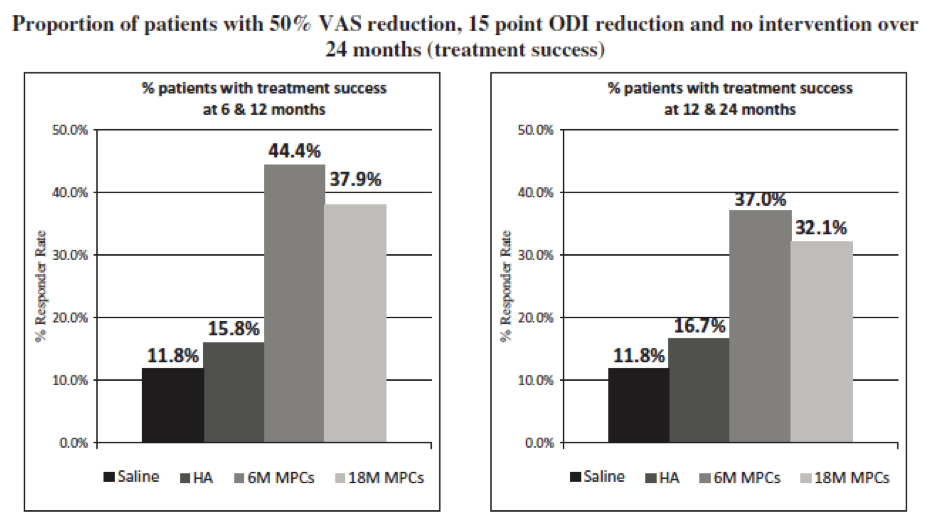
- Composite endpoint: Based on precedent and FDA feedback from our end-of-Phase 2 meeting, we developed a composite endpoint requiring at least a 50% improvement in low back pain, 15 point improvement in ODI and no treatment intervention (surgical or injection) that we believe would be sufficient to meet FDA’s requirements for approval. Utilizing this composite endpoint in a post-hoc analysis of Phase 2 data, separation between treatment and control arms was first seen at 3 months, maximal at 6 months, and sustained for at least 12 months. More specifically, the MPC-06-ID group, the 18 million MPC group, the HA control and the saline control groups had 44.4%, 37.9%, 15.8% and 11.8% of subjects meet the composite endpoint criteria at both 6 and 12 months. (MPC-06-ID vs. saline p<0.05). Moreover, the MPC-06-ID group had three times (3x) the proportion of patients achieving treatment success at both 12 and 24 months compared with saline controls (37.0% versus 11.8%, p=0.09).
- Forums
- ASX - By Stock
- MSB
- MPC-06-ID Phase 3 results countdown
MSB
mesoblast limited
Add to My Watchlist
3.91%
 !
$2.39
!
$2.39
MPC-06-ID Phase 3 results countdown
-
-
- There are more pages in this discussion • 214 more messages in this thread...
You’re viewing a single post only. To view the entire thread just sign in or Join Now (FREE)
Featured News
Add to My Watchlist
What is My Watchlist?
A personalised tool to help users track selected stocks. Delivering real-time notifications on price updates, announcements, and performance stats on each to help make informed investment decisions.
 (20min delay) (20min delay)
|
|||||
|
Last
$2.39 |
Change
0.090(3.91%) |
Mkt cap ! $3.020B | |||
| Open | High | Low | Value | Volume |
| $2.29 | $2.39 | $2.27 | $7.115M | 3.042M |
Buyers (Bids)
| No. | Vol. | Price($) |
|---|---|---|
| 53 | 90764 | $2.38 |
Sellers (Offers)
| Price($) | Vol. | No. |
|---|---|---|
| $2.39 | 110569 | 28 |
View Market Depth
| No. | Vol. | Price($) |
|---|---|---|
| 45 | 62614 | 2.380 |
| 41 | 88316 | 2.370 |
| 19 | 121690 | 2.360 |
| 17 | 166892 | 2.350 |
| 16 | 79054 | 2.340 |
| Price($) | Vol. | No. |
|---|---|---|
| 2.390 | 113645 | 29 |
| 2.400 | 205718 | 29 |
| 2.410 | 159274 | 18 |
| 2.420 | 257168 | 8 |
| 2.430 | 24759 | 2 |
| Last trade - 15.43pm 18/09/2025 (20 minute delay) ? |
Featured News
| MSB (ASX) Chart |