Last year the FDA’s Center for Drug Evaluation and Research (CDER) approved 37 novel drugs.
Biologics continue their ascendance, accounting for 41% of CDER’s approvals last year. This is the highest percentage of biologics to date.Antibody-based therapeutics— monoclonal antibodies (mAbs), bispecifics and antibody–drug conjugates (ADCs) — accounted for 30% of the approvals, another high-water mark (Fig. 3).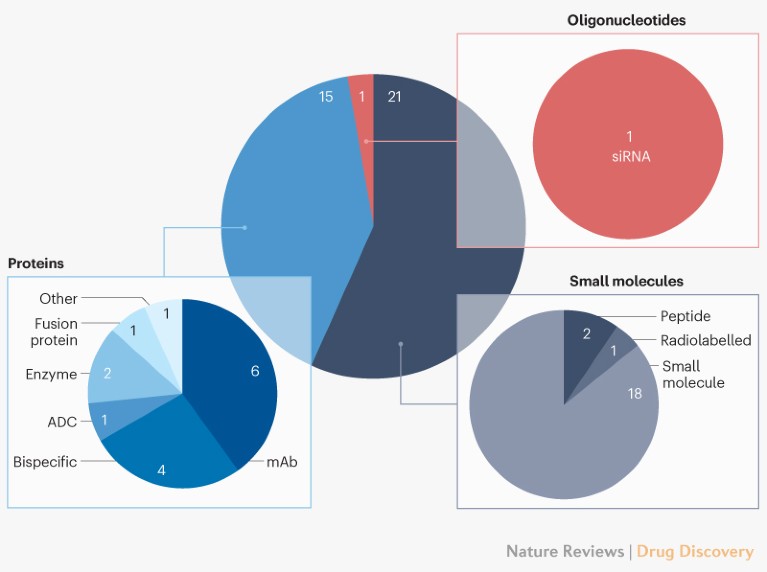
https://www.nature.com/articles/d41573-023-00001-3
NOT FINANCIAL ADVICE - DYOR
It should but in any case I'm long on this one so I don't pay too much attention on the sp daily close, rather on the weekly close or - significantly - on the monthly, which looks very promising as it shows that this move up is just starting with the RSI, the Stochastic and the MACD just turning and the sp just tagging the mid line of the Bollinger bands:
Chance are that at some stage there will be a retrace and possibly a tagging of the EMA200 on the daily, those are the buying opportunity however there is also the FOMO at play, consider the following:2022 FDA approvals











