Tryptamine Therapeutics
Who is TYP?
Tryptamine Therapeutics Limited (TYP) is a clinical-stage biotechnology company focused on developing innovative, scalable intravenous (IV) psilocin formulations. The company aims to address significant unmet medical needs through precision psychedelic therapies, primarily targeting eating disorders and chronic pain, with a first-to-market invention.
What are they bringing to market?
TYP is bringing TRP-8803 to market, a proprietary IV-infused psilocin formulation designed to overcome the limitations of oral psilocybin. This product offers rapid onset, controlled depth and duration of the psychedelic experience, and reduced treatment time. Positioned as a transformative and commercially scalable therapy, TRP-8803 has broad applicability and is supported by strong intellectual property. Notably, TRP-8803 represents a first-to-market opportunity with no direct clinical or commercial competitors.
How does it work?
TRP-8803 delivers psilocin, the active metabolite of psilocybin, directly into the bloodstream, allowing precise control over drug levels in patients. This method ensures a quicker onset of the psychedelic state (approximately 15 minutes), with a shorter, more manageable treatment duration of 1-2 hours. The therapy is also reversible in emergencies, enhancing its safety profile. By solving the IV-infusion challenge, TYP provides a significant advantage over oral administration competitors, optimizing both patient outcomes and clinic operations.
Clinical Data
Through partnerships with leading academic institutions, TYP has demonstrated positive effects with their oral psilocybin formulation, TRP-8802, in early clinical studies targeting conditions such as binge eating disorder, fibromyalgia, and irritable bowel syndrome (IBS). These studies have identified key safety and efficacy signals, allowing TYP to refine their clinical trial protocols and strengthen their intellectual property. These early successes have de-risked the development of their IV-infused psilocin formulation, TRP-8803, which is now poised for advanced Phase 2b/3 trials, offering a scalable solution with significant potential to improve patient outcomes.
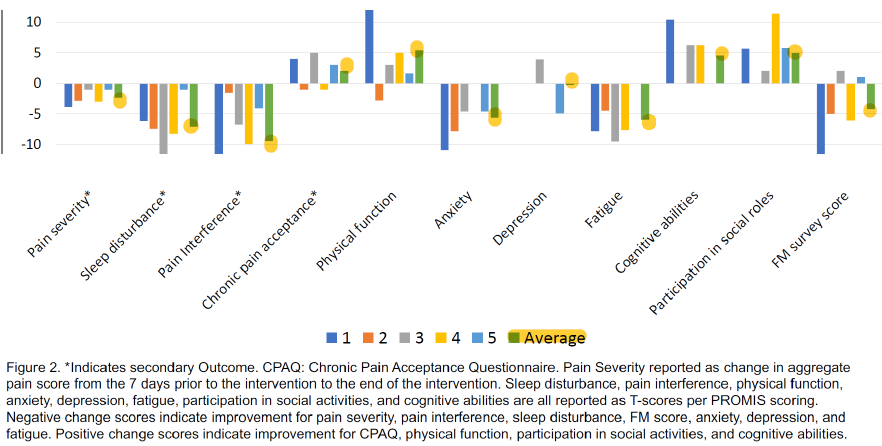
Fibromyalgia
Fibromyalgia, a condition characterized by widespread pain and significant disability, has emerged as a promising target for psilocybin therapy due to its potential analgesic effects. Psilocybin, primarily acting on the 5-HT2A receptor, has shown encouraging results in preliminary studies, with patients reporting various degrees of symptom relief and improved quality of life. Ongoing research, including advanced imaging studies, is focused on elucidating the neurobiological effects of psilocybin in fibromyalgia, highlighting the need for larger, controlled trials to confirm its therapeutic potential.
The results for TRP-8802 are impressive, with a 100% reduction in pain accompanied by improvements in pain interference, fatigue, and quality-of-life markers. Although this isn't my primary area of expertise, the literature I've reviewed suggests that the neurological effects of psilocybin are multifactorial, and it's encouraging to see these effects validated clinically. I'm particularly interested in how precise dosing and blood concentration control might further influence these outcomes in a larger dataset.
https://pubmed.ncbi.nlm.nih.gov/35001856/
https://pubmed.ncbi.nlm.nih.gov/38496341/
https://pubmed.ncbi.nlm.nih.gov/38983371/
https://pubmed.ncbi.nlm.nih.gov/37357750/
https://pubmed.ncbi.nlm.nih.gov/37199833/
https://pubmed.ncbi.nlm.nih.gov/37599279/
https://pubmed.ncbi.nlm.nih.gov/35254595/
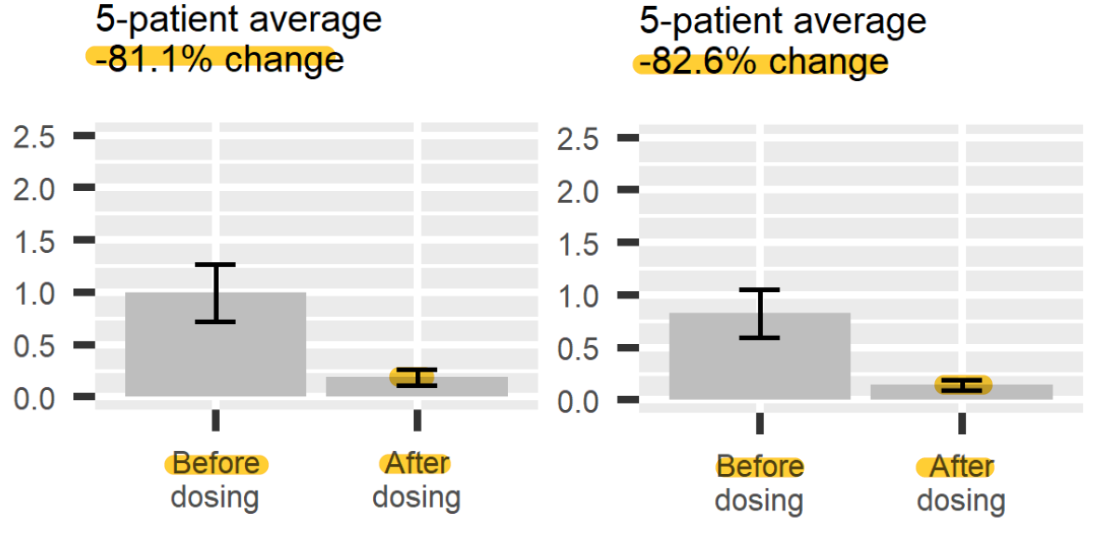
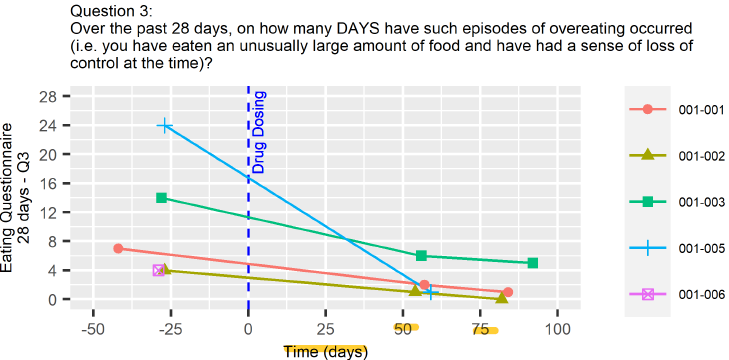
Binge Eating Disorder (BED)
Psilocybin is being investigated for its potential to treat Binge Eating Disorder (BED) by enhancing cognitive flexibility, improving body image, and normalizing reward processing. While animal studies have produced mixed results, early human trials have shown promising psychological benefits, suggesting that psilocybin-assisted therapy could effectively address the behavioral patterns underlying BED. However, further clinical research is required to fully validate its efficacy.
Notably, the trials of TRP-8802 demonstrated an excellent outcome with a >80% reduction in binge eating episodes, coupled with durable responses and significant decreases in anxiety and depression. What stands out is the low standard error in the dataset, despite the small patient population—an indicator of reliable data, as smaller whiskers generally denote more precise results. My background in Dietetics, both academically and professionally, clearly underscores the connection between emotional and mental wellbeing and dietary practices. It's encouraging to see the strong multifactorial relationship between reduced anxiety/depression scores and decreased eating episodes in this study.
https://pubmed.ncbi.nlm.nih.gov/35953488/
https://pubmed.ncbi.nlm.nih.gov/38123380/
https://pubmed.ncbi.nlm.nih.gov/37352816/
Inflammatory Bowel Syndrome (IBS)
Psilocybin has shown significant anti-inflammatory effects in studies using human intestinal models, positioning it as a promising candidate for managing IBS. By reducing key pro-inflammatory markers such as TNF-α, IFN-γ, and IL-6, psilocybin demonstrates potential in modulating inflammatory pathways. The efficacy of psilocybin is further enhanced when combined with agents like curcumin, presenting a novel therapeutic approach for IBS that merits further clinical investigation.
https://pubmed.ncbi.nlm.nih.gov/37623246/
https://pubmed.ncbi.nlm.nih.gov/38137946/
Competitors
Beckley Psytech - ELE-101 (IV Psilocin benzoate)
While Beckley Psytech is developing ELE-101 for Major Depressive Disorder, the focus and indication differ. In Phase I, ELE-101 demonstrated well-tolerated, high-intensity, short-duration psychedelic experiences with no serious adverse events. With a dose-proportional pharmacokinetic profile, ELE-101 suggests a brief clinical treatment window of approximately 2 hours. Phase IIa trials are currently underway. At the very least, this highlights that IV administration can work in humans, and the time to move on this invention is now.
Market Differentiation
TYP’s key differentiators include its IV-infusion technology, which allows for precise therapeutic blood concentrations, shorter treatment durations, and fewer side effects. TYP is also targeting indications with no current clinical or commercial competitors. As first-to-market, TYP has the potential to secure a significant market share, with the time unchallenged in the market being a critical factor in building value.
Value for TYP
With a current market cap of AUD $24M, TRP-8803, a Phase 2 asset, holds substantial value due to its efficacy across multiple indications and positive off-target effects. As the first-to-market solution for issues in traditional administration, TRP-8803’s swift progression from Phase 2a oral to Phase 2b IV, following a successful Phase 1 dose escalation trial, suggests that the current market cap may undervalue TYP. The recent FDA fast-track designation for treating fibromyalgia pain, which also highlights improvements in sleep quality, depression, and overall fibromyalgia symptoms, underscores the potential value not fully reflected in TYP’s market cap.
Conclusion
TYP fits the biotechnology criteria for a novel invention with excellent efficacy and few clinical or commercial competitors, to maximise value and minimise risk in the market.
 !
3.2¢
!
3.2¢
 (20min delay)
(20min delay)




