Your putting me on the hot seat here haha In my simpleton explanation, I believe CF33 is going to be ground breaking in the Cancer health space because it's safe and shown to kill multiple cancers in models. They are in multiple early trials treating various different cancers. FDA has also approved fast track for Bile Cancer and just last week management are hinting they are aiming for numerous more fast track designations. They aren't given out willy-nilly so big tick of approval.
I believe we have 1 CR in Vaccinia trial which Leslie said will soon be able to call 'Cured' because of the long time span of no returning cancer, I think almost 3years. Another big tick.
It is early days for CF33, so my views are based on all of the information available to me at the time. I wish you all the best with clinical success.
It was 430 days as of January 2024, which is a fantastic result for that patient. The issue I have is that it is one of few at this stage. Below is a table summarising all of the phase 1 clinical trials investigating OV therapy in advanced solid cancers by 2020. With regards to responders, CF33 is on the lower end of this table with a durable response rate (DRR; all CR and PR) of 9.7%. There have been more phase 1 trials completed to date; here are two examples with DRRs around 10-25% DRR (1,2). If you want to do some extra research, I recommend evaluating Reolysin. The FDA gave it fast track designation for breast cancer and orphan drug designation for ovarian and pancreatic, but it is yet to produce data that good enough for approval. None of these products have achieved clinical or commercial success.
I do not see anything special about CF33 when I compare it to this table.

1. https://www.nature.com/articles/s41417-023-00720-0
2. https://ascopubs.org/doi/10.1200/JCO.2023.41.16_suppl.9535
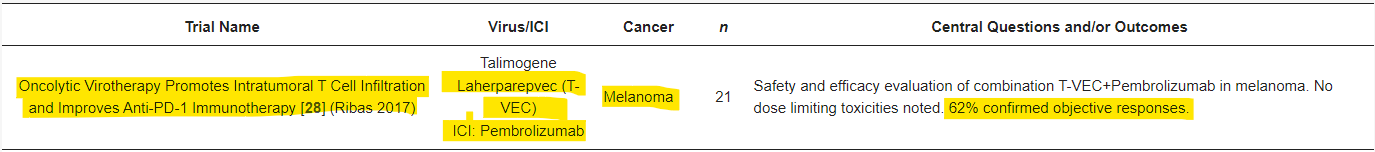
The complete response is excellent for IMU and CF33, because that cancer type has limited compounds that target it specifically - no clinical or commercial competitors. If it is a success clinically, you would have the best opportunity for a transaction. The issue with the two PRs in melanoma is that T-VEC is already approved for use as a single agent or in combination with immunotherapy - as it’s clearly stated, even if a 2nd to market is better they generate significantly less market capture. This means IMU needs to outperform T-VEC in melanoma, and even if you did, BP is going to struggle for market capture. Here is an example of T-VECs clinical efficacy with pembrolizumab.
Used in combo's it can search, recognise and destroy other cancers in the body. If someone is being treated for Breast cancer, then they develop Bile cancer. The virus already in your system could turn the Bile cancer tumour 'Hot' so your immune system would recognise it and kill the 2nd cancer when you aren't even being directly treated for it.
There is no data to suggest it can search, recognise, or destroy cancer. In fact, the IV data shows the total opposite. Based on the most recent update, almost 50% of the population treated intravenously have disease progression, meaning the drug is doing nothing. In the other 50%, it’s slowing the cancer, but not actively killing it. The use of the terminology “best” response indicates that some patients have progressed after their stable disease. There is also no combination data that proves what you are saying.

I think this leads into the Cancer Vaccine path which is still years or even decades away, but the fact that Imugene are treating hundreds of patients with little to no side effects means they can keep pushing the doses higher, big upside there.
Low side effects are extremely common among oncolytic viral therapies. There is literally nothing special about CF33 with regards to safety. Below is a systematic review consisting of 100’s of studies that supports this.

The next Vaccinia data cut due soon is extremely important because of this dose accelerating. It's still early days with 1 Complete Response so it's exciting to see if high doses leads to multiple CR sooner. Stay Tuned for another Tick.
I will stay tuned.
If you are contemplating investing in IMU for the business $ trade not science, then I think looking at the Keytruda patent expiring in 4years is a big catalyst. If I am not mistaken that means any BP will have access to Keytruda ending Merck's monopoly. So naturally Merck and other BP will be looking for new therapies to ENHANCE Keytrudas performance as their advertising point of difference. Cue Imugene already in combo trials and planning a multitude more. I believe this is where our best bet is selling/licencing our CF33 tech to BP who will use it in combo with Keytruda or other Cancer drugs which will provide enhanced cancer killing capabilities, without nasty side effects of Chemotherapy. Sound's like a milti billion $ deal right there waiting to happen. TICK TICK TICK!!!
Is there any clinical evidence to suggest that CF33 is combination with Pembrolizumab is demonstrating efficacy? My understanding is there are 0 responses. Something like this would excite me:
Phase 1b trial of T-VEC in combination with pembrolizumab.

The way that I see it is this. CF33 is a very early stage drug. The clinical efficacy has been significantly less than hoped for, so IMU management are attempting a higher dosing regimen in the hope that they find more responses.
The way that I come to a decision about investing in biotechnology may be different to you, but based on what I know so far, here is a short of what I think of CF33. Cholangiocarcinoma is a win for CF33, but there are some risks that I can see.
The IMU MC is roughly USD $460M. From my research, the global cholangiocarcinoma market is between USD $300M & $800M. Let’s assume it’s USD $800M.
If CF33 works well enough and is able to capture a realistic 10% of that market because not all patients with cholangiocarcinoma are relapsed/refractory to PD1/PDL1 therapies. The $ value of that market is $80M, which is ~5x less than the current MC of IMU.
Now, if it goes badly, and there are no or very few clinical responses, what will happen to CF33? We need to remember that the dose expansion is at the max dose and is including the patient group that has seen success from CF33.
A measure of risk vs reward is required here, and I am someone who thinks the risk does not equal the reward.
 (20min delay)
(20min delay)










