So Trump has the Covid-19
White House: Trump given Gilead Sciences' remdesivir, in addition to Regeneron drugWhite House officials said late Friday that President Donald Trump has received Gilead Sciences Inc.'s
GILD, -1.81% remdesivir
as part of his COVID-19 treatment plan. Remdesivir
was the first new drug to receive an emergency use authorization from the Food and Drug Administration during the pandemic as a treatment for severely ill COVID-19 patients. Clinical studies found it can reduce recovery times for hospitalized patients. Trump announced early Friday morning that he and the First Lady Melania Trump had tested positive for the coronavirus; since then several White House staffers have also tested positive for COVID-19. On Friday evening, Trump was given Regeneron Pharmaceuticals Inc.'s
REGN, -0.58% neutralizing antibody cocktail,
which is still being studied in clinical trials as a treatment for non-hospitalized patients with milder and more moderate forms of the disease. In a
letter shared early Saturday morning, Dr. Conley, the president's physician, said Trump had been moved to Walter Reed National Military Medical Center in Bethesda, Md., and he had also been given remdesivir. Conley said Trump does not require supplemental oxygen.
AUTHORISATION FOR USE OF REMDESIVIR IN AUSTRALIARemdesivir – information for cliniciansRemdesivir has provisional approval for the treatment of coronavirus disease 2019 (COVID-19) in adults and adolescents (aged 12 years and older, weighing at least 40kg) with pneumonia requiring supplemental oxygen. The decision to approve this medicine has been made based on limited data. More comprehensive evidence is required to be submitted.
Medical practitioners are invited to apply for a supply of remdesivir through the National Medical Stockpile. Gilead has donated a small supply to the Commonwealth government, which will be available for use in eligible patients. Eligible patients are generally adults who are hospitalised with moderate to severe COVID-19 pneumonia.
Read the eligibility criteria.
The use of remdesivir outside of clinical trials has been endorsed by the
National COVID-19 Clinical Evidence Taskforce.
An application form and more details about the supply from the National Medical Stockpile can be obtained by contacting
[email protected]PLEASE NOTE - REMDESIVIR IS SUPPLIED IN THIS PROGRAM FOR CLINICAL USE
ONLY – IT IS NOT FOR USE IN CLINICAL TRIALS
1
31 July 2020
Criteria for access to remdesivir from the National
Medical Stockpile
Treatment course is limited to 5 days for eligible patients. Applications must be signed by
treating consultant clinician (FRACP, FCICM or equivalent).
Inclusion Criteria:
• Informed consent provided by the patient,or patient’s legal representative.
• Age ≥ 18 years, or aged ≥ 12 and < 18 years of age weighing ≥ 40 kg.
• Hospitalised with confirmed SARS-CoV2 or known contact of confirmed case with syndrome
consistent with coronavirus disease (COVID-19) awaiting confirmation by diagnostic testing.
• Oxygen saturation (SpO2) ≤ 92% on room air and requiring supplemental oxygen
• Alanine aminotransferase (ALT) < 5 x upper limit of normal (ULN) by local laboratory measure
and/or ALT < 3 x ULN and bilirubin < 2 x ULN
Exclusion Criteria:
• Evidence of multiorgan failure including but not limited to coagulopathy (significant
thrombocytopenia), hepatic failure (elevated bilirubin) or renal failure (low urine output or
estimated glomerular filtration rate (eGFR) < 30 mL/min), or significant cardiomyopathy (low
cardiac output)
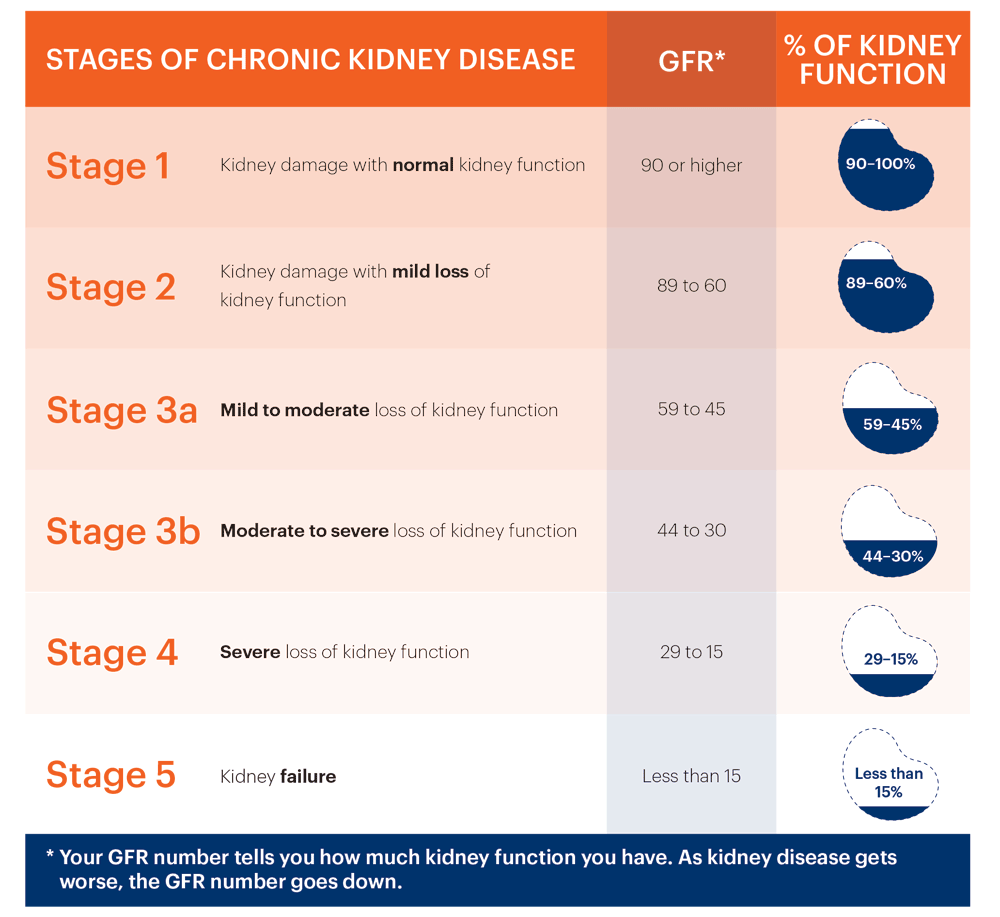
• Renal failure (eGFR < 30 mL/min or dialysis or continuous venovenous haemofiltration
• Mechanical ventilation for longer than 48 hours at time of application
• Receiving ECMO
• Known hypersensitivity to the study drug, the metabolites, or formulation excipient
Special Considerations
The clinical benefit of remdesivir is uncertain in the following scenarios. Clinicians should give
strong consideration to whether remdesivir is likely to benefit the patient in the following scenarios:
• Mechanical ventilation for less than 48 hours at time of application.
• Presence of an intercurrent illness which is likely to lead to the patient’s death within
one year
• Advanced age with limitations on activities of daily living.
• Need for more than a 5 day treatment course
For applications where these considerations apply the Department may contact the prescribing
clinician for further information.
The point of this thread is to remind those reading.......Starpharma has a formulation superior to Remdesivir
I will repost something written by me a few days ago......Hope you don't mind
Sources for these comments have come from internet, announcements, and analysts
Remdesivir has broad-spectrum antiviral activity. Current formulations of remdesivir are required to be administered intravenously due to the drug’s low solubility, with each infusion taking up to
two hours and
requiring daily administration for either 5 or 10 days.Intravenous Infusion
Some medications must be given by an
intravenous (
IV) injection or
infusion. This means they're sent directly into your vein using a needle or tube. In fact, the term “
intravenous” means “into the vein.” With
IV administration, a thin plastic tube called an
IV catheter is inserted into your vein

Gilead’s remdesevir has low water solubility, requires excipient {
Excipients are inert
pharmaceutical ingredients that
are used in product formulations.
Excipients may perform a variety of functional roles in the
pharmaceutical product. In the vast majority of cases,
excipients have limited (if any)
pharmacological activity, unlike the API [Active
Pharmaceutical Ingredients are the active ingredients in medications, carried by the excipient to make it soluble and be given as IV infusion} An excipient called Captisol is used in Gilead’s remdesivir formulation as a solubilizing agent due to the limited aqueous solubility of remdesivir. This excipient is renally cleared and can accumulate in patients with decreased renal function so the drug is not recommended to be administered in patients with eGFR of less than 30ml. It also requires daily administration for at least 5 days.
 https://btbcku.com/wp-content/uploa...ing-find-a-cure-for-the-coronavirus-1.mp4?_=1
https://btbcku.com/wp-content/uploa...ing-find-a-cure-for-the-coronavirus-1.mp4?_=1In contrast, Starpharma’s
DEP® remdesivir is a highly water-soluble nanoparticle formulation of remdesivir with controlled release properties, which would potentially allow for less frequent dosing and use in a non-hospital setting, such as aged-care. The solubility of DEP
® remdesivir is
100‑fold higher than standard remdesivir. The benefit of DEP
® remdesivir’s enhanced aqueous solubility is that it would enable subcutaneous injection rather than intravenous infusion, allowing for outpatient treatment and reducing the burden on hospitals.
Subcutaneous Injections
A subcutaneous injection is administered as a bolus (the administration of a discrete amount of medication, drug, or other compound within a specific time, generally within 1 - 30 minutes, in order to raise its concentration in blood to an effective level) into the subcutis, the layer of skin directly below the dermis and epidermis, collectively referred to as the cutis.
Subcutaneous injections are highly effective in administering medications such as insulin, morphine, diacetylmorphine and goserelin. Subcutaneous (as opposed to intravenous) injection of recreational drugs is referred to as "skin popping." Subcutaneous administration may be abbreviated as SC, SQ, sub-cu, sub-Q, SubQ, or subcut. Subcut is the preferred abbreviation for patient safety. Subcutaneous tissue has few blood vessels and so drugs injected here are for slow, sustained rates of absorption.
SUBCUTANEOUS INJECTION SITES

Comments by Jackie Fairley
“Given the limited treatment options available for COVID-19 patients, Starpharma has been actively reviewing development programs globally, and evaluating where Starpharma’s proprietary DEP® technology has potential to improve delivery, expand use or reduce frequency of dosing”.“The ability to deliver remdesivir via a long-acting, subcutaneous injection has the potential to expand its application outside hospitals, into settings like aged care, and also facilitate its use in countries with less developed healthcare systems. It would also improve patient convenience and reduce the burden on the healthcare system. We’re pleased to be able to utilise the DEP® platform to improve the delivery of this important antiviral medicine”.“The development of DEP® remdesivir is Starpharma’s 2nd program addressing COVID-19, and potentially future pandemics. This is separate to the development of its SPL7013 antiviral nasal spray for COVID-19, which was detailed in a market update last week”Advantage of using DEP® remdesivir
A subcutaneous formulation would be desirable in settings such as aged care, nursing homes and for less developed countries which typically have less developed healthcare systems and shortage of resources such as hospital beds to cope with the COVID-19 pandemic. It can also be given to COVID-19 patients who do not require hospitalization, to speed up their recovery.
Advantage of Starpharma's DEP® remdesivir over Gilead's Veklury (Remdesivir)
- Can be injected subcutaneously and not infused intravenously
- Does not need to be used in hospital settings
- Can be administered in aged care, nursing homes
- Can be freely available in less developed countries which have less developed healthcare systems and shortage of resources such as hospital beds to cope with COVID-19 pandemic
- Can be administered to COVID-19 patients who do not require hospitalisation
Gilead's Veklury (Remdesivir) is still patent protected in most jurisdictions. For progression of DEP® remdesivir, Starpharmat would need to partner with Gilead. One must assume Starpharma is currently in discussions with Gilead. Clinical trials would need to be conducted.
While in early stages of development, the development path for DEP-remdesivir is likely to be accelerated under Emergency use Authorisation pathways in US and elsewhere for COVID-19 therapies. Clinical data would be required in humans (potentially healthy volunteers and non-hospitalised COVID-19 patients) to assess whether pharmacokinetics, safety, tolerability and plasma drug levels of the drug is comparable to Gileads IV remdesivir formulation
Could you imagine if Gilead say to Starpharma......
"look we do not want you as partners.....we want to buy the your formulation outright
Here is an offer you cannot refuse US$500 million
What do you say"
Look forward to some more market sensitive announcementsI am sure everyone shares my current excitement and enthusiasm for Starpharma
 !
9.5¢
!
9.5¢
 (20min delay)
(20min delay)




